
REF 09N77-095
51-608445/R4
NOTE: Changes highlighted
For use under an Emergency Use Authorization (EUA) Only
Instructions for Use
INTRODUCTION
This Emergency Use Authorization (EUA) package insert must be read carefully prior to use. EUA package insert instructions must be followed accordingly. Reliability of EUA assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
NAME
Abbott RealTime SARS-CoV-2
INTENDED USE
The Abbott RealTime SARS-CoV-2 assay is a real-time (rt) reverse transcriptase (RT) polymerase chain reaction (PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in nasal swabs, self-collected at a health care location or collected by a healthcare worker and nasopharyngeal (NP) and oropharyngeal (OP) swabs, and bronchoalveolar lavage fluid (BAL) collected by a healthcare worker, from individuals suspected of COVID-19 by their healthcare provider.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity tests.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
The Abbott RealTime SARS-CoV-2 assay is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures. The Abbott RealTime SARS-CoV-2 assay is only for use under the Food and Drug Administration’s Emergency Use Authorization.
SUMMARY AND EXPLANATION OF THE TEST
The Abbott RealTime SARS-CoV-2 assay is real-time reverse transcription polymerase chain reaction (rRT-PCR) test on the Abbott m2000 System. The SARS-CoV-2 primer and probe sets are designed to detect RNA respiratory specimens collected from patients who are suspected of COVID-19 by their health care provider.
BIOLOGICAL PRINCIPLES OF THE PROCEDURE
The Abbott RealTime SARS-CoV-2 assay consists of 2 reagent kits:
- Abbott RealTime SARS-CoV-2 Amplification Reagent Kit
- Abbott RealTime SARS-CoV-2 Control Kit
The Abbott RealTime SARS-CoV-2 assay is a dual target assay for the RdRp and N genes.
An RNA sequence that is unrelated to the SARS-CoV-2 target sequence is introduced into each specimen at the beginning of sample preparation. This unrelated RNA sequence is simultaneously amplified by RT-PCR and serves as an internal control (IC) to demonstrate that the process has proceeded correctly for each sample.
The Abbott RealTime SARS-CoV-2 assay detects the SARS-CoV-2 virus and IC target sequences through the use of target-specific fluorescent-labeled oligonucleotide probes. The probes do not generate a signal unless they are specifically bound to the amplified product. The two SARS-CoV-2-specific probes are labeled with the same fluorophore and the IC-specific probe is labeled with a different fluorophore, thus allowing for simultaneous detection of both SARS-CoV-2 and IC amplified products in the same reaction well.
The Abbott RealTime SARS-CoV-2 assay is performed on the Abbott m2000 System consisting of a sample preparation unit, the Abbott m2000sp, and an amplification and detection unit, the Abbott m2000rt. Application parameters specific to the Abbott RealTime SARS-CoV-2 assay are contained on an assay-specific application specification file, distributed electronically, stored on portable media and loaded onto the Abbott m2000sp and Abbott m2000rt instruments.
Sample Preparation
The Abbott m2000sp provides automated sample preparation using a magnetic microparticle-based protocol and reagents (Abbott mSample Preparation SystemDNA) to process respiratory specimens.
During the sample preparation protocol, SARS-CoV-2 virions are disrupted by guanidine isothiocyanate, nucleic acids are captured on the magnetic microparticles, and inhibitors and unbound sample components are removed by washing steps. The bound nucleic acids are eluted off the microparticles with buffer and transferred to a 96 deep-well plate. The nucleic acids are then ready for amplification. The Internal Control (IC) is introduced into each specimen at the beginning of the sample preparation process to demonstrate that the process was completed correctly for each specimen and control.
A positive control and a negative control are processed from the start of sample preparation for each test order to evaluate run validity.
The purpose of sample preparation is to extract and concentrate the target nucleic acids to make the target accessible for amplification, and to remove potential inhibitors of amplification from the extract.
The Abbott mSample Preparation SystemDNA uses magnetic particle technology to capture nucleic acids and washes the particles to remove unbound sample components. The bound nucleic acids are eluted and transferred to a 96 deep-well plate. The nucleic acids are then ready for amplification. The IC is taken through the entire sample preparation procedure along with the controls and specimens.
The Abbott m2000sp automated instrument system is used to prepare samples for the Abbott RealTime SARS-CoV-2 assay. The Abbott m2000sp provides automated sample eluate transfer and reaction assembly in the Abbott 96-Well Optical Reaction Plate.
Reagent Preparation and Reaction Plate Assembly
The Abbott m2000sp combines the Abbott RealTime SARS-CoV-2 assay amplification reagent components (SARS-CoV-2 Oligonucleotide Reagent, Thermostable rTth Polymerase Enzyme, and Activation Reagent). The Abbott m2000sp dispenses the resulting master mix to the Abbott 96-Well Optical Reaction Plate along with aliquots of the nucleic acid samples prepared by the Abbott m2000sp. The plate is ready, after manual application of the optical seal, for transfer to the Abbott m2000rt.
Amplification
During the amplification reaction on the Abbott m2000rt, the target RNA is converted to cDNA by the reverse transcriptase activity of the thermostable rTth DNA polymerase. First, the SARS-CoV-2 and IC reverse primers anneal to their respective targets and are extended during a prolonged incubation period. After a denaturation step, in which the temperature of the reaction is raised above the melting point of the double-stranded cDNA : RNA product, a second primer anneals to the cDNA strand and is extended by the DNA polymerase activity of the rTth enzyme to create a double-stranded DNA product.
During each round of thermal cycling, amplification products dissociate to single strands at high temperature allowing primer annealing and extension as the temperature is lowered. Exponential amplification of the product is achieved through repeated cycling between high and low temperatures, resulting in a billion-fold or greater amplification of target sequences. Amplification of the three targets (SARS-CoV-2 RdRp, SARS-CoV-2 N, and IC) takes place simultaneously in the same reaction.
The target sequences for the Abbott RealTime SARS-CoV-2 assay are in the SARS-CoV-2 RdRp and N genes of the SARS-CoV-2 genome. The selected target sequences are highly conserved and also specific to this strain of coronavirus.
The IC target sequence is derived from the hydroxypyruvate reductase gene from the pumpkin plant, Cucurbita pepo, and is delivered in an Armored RNA® particle that has been diluted in negative human plasma.
Detection
During the read cycles of amplification on the Abbott m2000rt, the temperature is lowered further to allow fluorescent detection of amplification products as the SARS-CoV-2 and IC probes anneal to their targets (real-time fluorescence detection). The SARS-CoV-2 probes have a fluorescent moiety that is covalently linked to the 5′ end and has a quencher molecule at its 3′ end. In the absence of target sequences, the probes adopt a conformation that brings the quencher close enough to the excited fluorophore to absorb its energy before it can be fluorescently emitted. When the probe binds to its complementary sequence in the target, the fluorophore and the quencher are held apart, allowing fluorescent emission and detection. The IC probe is a single-stranded DNA oligonucleotide with a fluorophore at the 5′ end and a quencher at the 3′ end. In the absence of IC target sequences, probe fluorescence is quenched. In the presence of IC target sequences, probe hybridization to complementary sequences separates the fluorophore and the quencher and allows fluorescent emission and detection. The SARS-CoV-2 and IC specific probes are each labeled with a different fluorophore, thus allowing for simultaneous detection of both amplified products.
PREVENTION OF NUCLEIC ACID CONTAMINATION
The possibility of nucleic acid contamination is minimized because:
- Reverse transcription, PCR amplification, and oligonucleotide hybridization occur in a sealed Abbott 96-Well Optical Reaction Plate.
- Detection is carried out automatically without the need to open the Abbott 96-Well Optical Reaction Plate.
- Pipettes with aerosol barrier tips or disposable transfer pipettes are used for all pipetting. The disposable pipettes or pipette tips are discarded after use.
- Separate, dedicated areas are used to perform the Abbott RealTime SARS-CoV-2 assay. Refer to the SPECIAL PRECAUTIONS section of this package insert.
REAGENTS
- Abbott RealTime SARS-CoV-2 Internal Control
(4 vials, 1.2 mL per vial)- < 0.01% noninfectious Armored RNA with internal control sequences in negative human plasma. Negative human plasma tested and found to be non-reactive by appropriate FDA-licensed, approved, or cleared tests for antibody to HCV, antibody to HIV-1, antibody to HIV-2, HIV-1 Ag, HBsAg, and Syphilis. The material is also tested and found to be negative by appropriate FDA-licensed, approved, or cleared PCR methods for HIV RNA, HCV RNA, and HBV DNA. Preservatives: 0.1% ProClin® 300 and 0.15% ProClin 950.
- Abbott RealTime SARS-CoV-2 Amplification Reagent Pack (List No. 9N77)
(4 packs, 24 tests/pack)- 1 bottle (0.141 mL) Thermostable rTth Polymerase Enzyme (2.9 to 3.5 Units/μL) in buffered solution.
- 1 bottle (1.0 mL) SARS-CoV-2 Amplification Reagent containing synthetic oligonucleotides (6 primers and 3 probes), and dNTPs in a buffered solution with a reference dye. Preservative: 0.10% ProClin 300 and 0.15% ProClin 950.
- 1 bottle (0.400 mL) Activation Reagent. 30 mM manganese chloride solution. Preservatives: 0.10% ProClin 300 and 0.15% ProClin 950.
Abbott RealTime SARS-CoV-2 Control Kit (List No. 09N77-085)
- Abbott RealTime SARS-CoV-2 Negative Control
(8 vials, 1.3 mL per vial) Contains 1.0% ammonium sulfate and 7.9% detergent in a buffer solution. - Abbott RealTime SARS-CoV-2 Positive Control
(8 vials, 1.3 mL per vial) Contains non-infectious, recombinant Sindbis virus containing SARS-CoV-2 RNA sequences, 1.0% ammonium sulfate, and 7.9% detergent in a buffer solution.
WARNINGS AND PRECAUTIONS
For Use Under An Emergency Use Authorization Only.
This assay is only for in vitro diagnostic use under the FDA Emergency Use Authorization.
For Prescription Use Only.
- This test has not been FDA cleared or approved;
- This test has been authorized by FDA under an EUA for use by authorized laboratories;
- This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; and
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Safety Precautions
- Refer to the Abbott m2000sp and Abbott m2000rt Operations Manuals, Hazard Section, for instructions on safety precautions.
- Important information regarding the safe handling, transport and disposal of this product is contained in the Safety Data Sheet.
CAUTION: This preparation contains human sourced and/or potentially infectious components. Components sourced from human blood have been tested and found to be nonreactive by appropriate FDA-licensed, approved, or cleared tests for antibody to HCV, antibody to HIV-1, antibody to HIV-2, HIV-1 Ag, HBsAg, and Syphilis. The material is also tested and found to be negative by appropriate FDA-licensed, approved, or cleared PCR methods for HIV RNA, HCV RNA, and HBV DNA. No known test method can offer complete assurance that products derived from human sources or inactivated microorganisms will not transmit infection. These reagents and human specimens should be handled as if infectious using laboratory safety procedures, such as those outlined in Biosafety in Microbiological and Biomedical Laboratories,1 OSHA Standards on Bloodborne Pathogens,2 CLSI Document M29-A4,3 and other appropriate biosafety practices.4 Therefore all human sourced materials should be considered infectious.
These precautions include, but are not limited to, the following:
- Wear gloves when handling specimens or reagents.
- Do not pipette by mouth.
- Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in areas where these materials are handled.
- Clean and disinfect spills of specimens by including the use of a tuberculocidal disinfectant such as 1.0% sodium hypochlorite or other suitable disinfectant.1
- Decontaminate and dispose of all potentially infectious materials in accordance with local, state, and federal regulations.4
Components of the Abbott RealTime SARS-CoV-2 Internal Control, Oligonucleotide Reagent, and Activation Reagent contain the following components:
2-Methyl-4-isothiazol-3-one:
- Reaction mass of: 5-chloro-2-methyl-4-isothiazolin-3-one (EC no. 247-500-7) and 2-methyl-2H-isothiazol-3-one (EC no. 220-239-6)(3:1)
- Reaction mass of: 5-chloro-2-methyl-4-isothiazolin-3-one (EC no. 247-500-7) and 2-methyl-4-isothiazolin-3-one (EC no. 220-239-6)(3:1)
Potassium Hydroxide
The following warnings apply:

* Not applicable where regulation EC 1272/2008 (CLP) has been implemented.
Important information regarding the safe handling, transport, and disposal of this product is contained in the Safety Data Sheet. Safety Data Sheets are available from your Abbott Representative.
SPECIAL PRECAUTIONS
As with any test procedure, good laboratory practice is essential to the proper performance of this assay. Due to the high sensitivity of this test, care should be taken to keep reagents and amplification mixtures free of contamination.
- For in vitro diagnostic use under Emergency Use Authorization only.
- Positive results are indicative of the presence of SARS-CoV-2 RNA.
- Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
- All patient samples should be handled as if infectious, using good laboratory procedures as outlined in Biosafety in Microbiological and Biomedical Laboratories1 and in the CLSI Document M29-A4.3 Only personnel proficient in handling infectious materials and the use of the Abbott RealTime SARS-CoV-2 assay and the Abbott m2000 System should perform this procedure.
Handling Precautions for Specimens
- The Abbott RealTime SARS-CoV-2 assay is only for use with respiratory specimens that have been handled and stored as described in the SPECIMEN COLLECTION, STORAGE, AND TRANSPORT TO THE TEST SITE section.
- Inadequate or inappropriate specimen collection, storage, and transport are likely to yield false test results. Training in specimen collection is highly recommended due to the importance of specimen quality. Refer to CLSI MM13-A 5 as an appropriate resource.
- During preparation of samples, compliance with good laboratory practices is essential to minimize the risk of cross-contamination between samples and the inadvertent introduction of ribonucleases (RNases) into samples during and after the extraction procedure.
- Proper aseptic technique should always be used when working with RNA.
- Amplification technologies such as PCR are sensitive to accidental introduction of product from previous amplification reactions. Incorrect results could occur if either the clinical specimen or the reagents used become contaminated by accidental introduction of even a few molecules of amplification product. Measures to reduce the risk of contamination in the laboratory include physically separating the activities involved in performing PCR in compliance with good laboratory practices.
Work Areas
The m2000sp and the m2000rt instruments may be operated in the same location. The use of 2 dedicated areas (Sample Preparation Area and Amplification Area) within the laboratory is recommended when performing the Abbott RealTime SARS-CoV-2 assay.
The Sample Preparation Area is dedicated to processing samples (specimens and Abbott RealTime SARS-CoV-2 Controls) and to adding processed samples and controls to the 96‑Well Optical Reaction Plate. All reagents used in the Sample Preparation Area should remain in this dedicated area at all times. Laboratory coats, pipettes, pipette tips, and vortexers used in the Sample Preparation Area must remain in this area and not be moved to the Amplification Area. Do not bring amplification product into the Sample Preparation Area.
The Amplification Area is dedicated to the amplification and detection of amplified product. Laboratory coats and equipment used in the Amplification Area must remain in this area and not be moved to the Sample Preparation Area.
- Components contained within a kit are intended to be used together. Do not mix components from different kit lots. For example, do not use the negative control from control kit lot X with the positive controls from control kit lot Y.
- Do not use kits or reagents after the expiration dates shown on kit labels.
- Work area and instrument platforms must be considered potential sources of contamination. Change gloves after contact with potential contaminants (specimens, eluates, and/or amplified product) before handling unopened reagents, negative control, positive controls, or specimens. Refer to the Abbott m2000sp and Abbott m2000rt Operations Manuals for instrument cleaning procedures.
- If the Abbott m2000sp instrument run is aborted, dispose of all commodities and reagents according to the Abbott m2000sp Operations Manual.
- If the Abbott m2000sp master mix addition protocol is aborted, seal the Abbott 96-Well Optical Reaction Plate in a sealable plastic bag and dispose according to the Abbott m2000sp Operations Manual, Hazards section, along with the gloves used to handle the plate.
- If the Abbott m2000rt instrument run is interrupted or aborted, seal the Abbott 96-Well Optical Reaction Plate in a sealable plastic bag and dispose according to the Abbott m2000rt Operations Manual along with the gloves used to handle the plate.
- Decontaminate and dispose of all potentially biohazardous materials in accordance with local, state, and federal regulations.4 All materials should be handled in a manner that minimizes the chance of potential contamination of the work area.
NOTE: Autoclaving the sealed Reaction Plate will not degrade the amplified product and may contribute to the release of the amplified product by opening the sealed plate. The laboratory area can become contaminated with amplified product if the waste materials are not carefully handled and contained.
Aerosol Containment
To reduce the risk of nucleic acid contamination due to aerosols formed during manual pipetting, aerosol barrier pipette tips must be used for all manual pipetting. The pipette tips must be used only 1 time. Clean and disinfect spills of specimens and reagents as stated in the Abbott m2000sp and Abbott m2000rt Operations Manuals.
Contamination and Inhibition
The following precautions should be observed to minimize the risks of RNase contamination, cross-contamination between samples, and inhibition:
- Wear appropriate personal protective equipment at all times.
- Use powder-free gloves.
- Change gloves after having contact with potential contaminants (such as specimens, eluates, and/or amplified product).
- To reduce the risk of nucleic acid contamination due to aerosols formed during pipetting, pipettes with aerosol barrier tips must be used for all pipetting. The length of the tip should be sufficient to prevent contamination of the pipette barrel. While pipetting, care should be taken to avoid touching the pipette barrel to the inside of the sample tube or container. The use of extended aerosol barrier pipette tips is recommended.
- Change aerosol barrier pipette tips between ALL manual liquid transfers.
- The Abbott mSample Preparation SystemDNA reagents are single use only. Use new reagent troughs or vessels, reaction vessels, and newly opened reagents for every new Abbott RealTime SARS-CoV-2 assay run. At the end of each run, discard all remaining reagents from the worktable as stated in the Abbott m2000sp Operations Manual and the Abbott mSample Preparation SystemDNA product information sheet.
STORAGE INSTRUCTIONS
Abbott RealTime SARS-CoV-2 Amplification Reagent Kit (List No. 09N77-095)

- Abbott RealTime SARS-CoV-2 Amplification Reagent Packs and Internal Control (IC) vials must be stored at – 25 to – 15°C when not in use. Care must be taken to separate the Abbott RealTime SARS-CoV-2 Amplification Reagent Pack that is in use from direct contact with samples and controls.
Abbott RealTime SARS-CoV-2 Control Kit (List No. 09N77-085)

- The Abbott RealTime SARS-CoV-2 Negative and Positive Controls must be stored at – 25 to – 15°C.
SHIPPING CONDITIONS
- Abbott RealTime SARS-CoV-2 Amplification Reagent Kit: Ship on dry ice.
- Abbott RealTime SARS-CoV-2 Control Kit: Ship on dry ice.
If you receive reagents that are in a condition contrary to label recommendation, or that are damaged, contact your Abbott Representative.
INDICATION OF INSTABILITY OR DETERIORATION OF REAGENTS
When a positive or negative control value is out of the expected range, it may indicate deterioration of the reagents. Associated test results are invalid and samples must be retested.
SPECIMEN COLLECTION, STORAGE, AND TRANSPORT TO THE TEST SITE
Human respiratory specimens may be used with the Abbott RealTime SARS-CoV-2 assay. Refer to the CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19)6 https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html or the FDA FAQs on Diagnostic Testing for SARS-CoV-2 https://www.fda.gov/medical-devices/emergency-situations-medical-devices/faqs-diagnostic-testing-sars-cov-2
An Abbott multi-Collect Specimen Collection Kit (List No. 09K12-01 (CE), 09K12-02 (CE), 09K12-03 or 09K12-04) or Abbott Universal Collection Kit (List No. 09N77-055) can be used for the transport of nasopharyngeal swab specimens or the collection and transport of nasal and oropharyngeal swab specimens from the collection site to the testing laboratory. Neither the swab nor the transfer pipette are authorized for nasopharyngeal specimen collection. The Transport Tube contains Specimen Transport Buffer which is used to stabilize nucleic acid until sample preparation. Transport and store transport tube at 2 to 25°C for up to 48 hours. If delivery and processing exceed 48 hours, specimens should be transported in dry ice and once in laboratory frozen at –70°C or colder.
- Discard disposable transfer pipette (if present); it is not required for nasal or oropharyngeal swab specimen collection.
- Remove the sterile swab from the wrapper, taking care not to touch swab tip or lay it down on any surface. Do not pre-wet swab.
- Collect patient specimen per CDC guidelines.6
- Handle the cap and tube carefully to avoid contamination, including the outside of the transport tube and cap. If necessary, change gloves.
- Unscrew the transport tube cap and immediately place the specimen collection swab into the transport tube so that the white tip is down.
- Carefully break the swab at the scored line on the shaft; use care to avoid splashing of contents.
- Recap the transport tube. Ensure the cap seals tightly. The cap must be tight or leakage may occur.
- Label the transport tube with sample identification information, including date of collection using an adhesive label. It is recommended that each tube be placed in an individual, sealable bag prior to transport.
- Discard disposable transfer pipette (if present) and the swab; they are not authorized for nasopharyngeal swab specimen collection.
- Collect patient specimen per CDC guidelines.6
- Handle the cap and tube carefully to avoid contamination, including the outside of the transport tube and cap. If necessary, change gloves.
- Unscrew the transport tube cap and immediately place the specimen collection swab into the transport tube so that the swab tip is down.
- If necessary, carefully break any swab shaft that protrudes out of the tube; use care to avoid splashing of contents.
- Recap the transport tube. Ensure the cap seals tightly. The cap must be tight or leakage may occur.
- Label the transport tube with sample identification information, including date of collection using an adhesive label. It is recommended that each tube be placed in an individual, sealable bag prior to transport.
For domestic and international shipments, specimens must be packaged, shipped, and transported according to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulation. Follow shipping regulations for UN 3373 Biological Substance, Category B when sending potential SARS-CoV-2 specimens.
INSTRUMENT PROCEDURE
The Abbott RealTime SARS-CoV-2 application specification files must be installed on the Abbott m2000sp and Abbott m2000rt instruments from the Abbott RealTime SARS-CoV-2 Application Specification (List No. 09N77-010 or higher) prior to performing the assay. For a detailed description of how to perform an Abbott m2000sp instrument and Abbott m2000rt instrument protocol, refer to the Abbott m2000sp and Abbott m2000rt Operations Manuals, Operating Instructions sections.
ABBOTT REALTIME SARS-COV-2 ASSAY PROCEDURE
This package insert contains instructions for running the Abbott RealTime SARS-CoV-2 assay.
Materials Provided
- Abbott RealTime SARS-CoV-2 Amplification Reagent Kit (List No. 09N77-095)
Materials Required But Not Provided
- Abbott mSample Preparation SystemDNA (List No. 06K12-24)
- Abbott RealTime SARS-CoV-2 Application Specification (List No. 09N77-010 or higher)
- Abbott RealTime SARS-CoV-2 Control Kit (List No. 09N77-085)
Other Optional Materials
- Abbott multi-Collect Specimen Collection Kit (List No. 09K12-01, 09K12-02, 09K12-03 or 09K12-04)
NOTE: List No. 09K12-01 and 09K12-02 are CE-marked.
- Abbott m2000sp Instrument (m2000sp software version 8.1 or higher)
- Abbott m2000sp Operations Manual (List No. 09K20-009 or higher)
- Abbott mSample Preparation SystemDNA (List No. 06K12-24)
- Abbott RealTime SARS-CoV-2 Application Specification (List No. 09N77-010 or higher)
- 5 mL Reaction Vessels (12 x 75 mm) (List No. 4J71-20)
- Master Mix Tubes (List No. 04J71-80)
- Amplification Reagent Pack Caps (List No. 3N20-01) (Optional)
- Transport Tubes (List No. 04J71-81)
- 200 mL Reagent Vessels (List No. 4J71-60)
- Abbott 96-Well Optical Reaction Plate (List No. 04J71-70)
- Abbott 96-Deep-Well Plate (List No. 04J71-30)
- Abbott Splash-Free Support Base (List No. 09K31-01)
- 200 μL and 1000 μL Disposable Tips for Abbott m2000sp (List No. 4J71-17 and 4J71-10)
- Abbott Optical Adhesive Cover (List No. 04J71-75)
- Abbott Adhesive Cover Applicator (List No. 9K32-01)
- Biohazard bags (List No. 4J71-45)
- Sample racks
- USP Grade 190 to 200 Proof Ethanol (95 to 100% Ethanol).
Do not use ethanol that contains denaturants. - Calibrated precision pipettes capable of delivering 20 μL to 1000 μL
- 20 μL to 1000 μL aerosol barrier pipette tips for precision pipettes.
Other Materials
- Biological safety cabinet approved for working with infectious materials
- Sealable plastic bags
- RNase-free water (Eppendorf or equivalent)†
- 1.7 mL molecular biology grade microcentrifuge tubes (Dot Scientific, Inc. or equivalent)†
- Cotton Tip Applicators (Puritan or equivalent)†
† Note: These 3 items are used in the procedure for Monitoring the Laboratory for the Presence of Contamination.
Refer to the QUALITY CONTROL PROCEDURES section of this package insert.
- Abbott m2000rt Instrument (m2000rt software version 8.1 or higher)
- Abbott m2000rt Operations Manual (List No. 06N03-009 or higher)
- Abbott RealTime SARS-CoV-2 Application Specification (List No. 09N77-010 or higher)
- Abbott m2000rt Optical Calibration Kit (List No. 4J71-93)
Other Materials
- Sealable plastic bags
Procedural Precautions
- Read the instructions in this package insert carefully before processing samples.
- The Abbott RealTime SARS-CoV-2 Negative Control and Positive Control vials are intended for single-use only and should be discarded after use.
- Use aerosol barrier pipette tips or disposable pipettes only one time when pipetting specimens or IC. To prevent contamination to the pipette barrel while pipetting, care should be taken to avoid touching the pipette barrel to the inside of the sample tube or container. The use of extended aerosol barrier pipette tips is recommended.
- Monitoring procedures for the presence of amplification product can be found in the QUALITY CONTROL PROCEDURES section in this package insert.
- To reduce the risk of nucleic acid contamination, clean and disinfect spills of specimens by including the use of a tuberculocidal disinfectant such as 1.0% sodium hypochlorite or other suitable disinfectant.
- The Abbott RealTime SARS-CoV-2 Controls must be prepared in conjunction with the specimens to be tested. The use of the Abbott RealTime SARS-CoV-2 Controls is integral to the performance of the Abbott RealTime SARS-CoV-2 assay. Refer to the QUALITY CONTROL PROCEDURES section of this package insert for details.
ASSAY PROTOCOL
For a detailed description of how to perform an Abbott m2000sp instrument and Abbottm2000rt instrument protocol, refer to the Abbott m2000sp and Abbott m2000rt Operations Manuals, Operating Instructions sections.
Laboratory personnel must be trained to operate the Abbott m2000sp and Abbott m2000rt instruments. The operator must have a thorough knowledge of the applications run on the instruments and must follow good laboratory practices.
Sample Preparation Area
- Thaw assay controls and IC at 15 to 30°C or at 2 to 8°C.
- Once thawed, assay controls and IC can be stored at 2 to 8°C for up to 24 hours before use.
- Vortex each assay control 3 times for 2 to 3 seconds before use. Ensure that the contents of each vial are at the bottom after vortexing by tapping the vials on the bench to bring liquid to the bottom of the vial. NOTE: Avoid excessive foaming.
- Select amplification reagent packs to be used in the run. Refer to the Abbott m2000sp Operations Manual (List No. 09K20 version 9 or higher), Operating Instructions section, for instructions pertaining to amplification reagent pack inventory management. All amplification reagent packs used in runs of greater than 24 reactions must have the same lot number. Thaw amplification reagents at 15 to 30°C or at 2 to 8°C and store at 2 to 8°C until required for the amplification master mix procedure. Once thawed, the amplification reagents can be stored at 2 to 8°C for up to 24 hours if not used immediately.
The following table shows the number of sample preparation reagents and internal control vials needed based on the number of reactions.

Abbott m2000sp Procedure
3. Gently invert the Abbott mSample Preparation bottles to ensure a homogeneous solution. If crystals are observed in any of the reagent bottles upon opening, allow the reagent to equilibrate at room temperature until the crystals disappear. Do not use the reagents until the crystals have dissolved. Add USP Grade 190 to 200 Proof Ethanol (95 to 100% Ethanol) to the mLysisDNA, mWash1DNA, and mWash2DNA bottles as indicated below. Do not use ethanol that contains denaturants.
- Add 35 mL ethanol to each bottle of mLysisDNA being used.
- Add 23 mL ethanol to each bottle of mWash1DNA being used.
- Add 70 mL ethanol to each bottle of mWash2DNA being used.
4. Vortex each IC 3 times for 2 to 3 seconds before use.
5. Use a calibrated precision PIPETTE DEDICATED FOR INTERNAL CONTROL USE ONLY to add 1200 μL of IC to each bottle of mLysis Buffer. Mix by gently inverting the container 5 to 10 times to minimize foaming and pour the contents into the appropriate reagent vessels per the table above. When pouring in 2 bottles of the mLysisDNA with the Ethanol and IC, fill reagent vessel no higher than the fill line where the top of the reagent label is placed. Ensure bubbles or foam are not generated in the reagent vessels; if present, remove with a sterile pipette tip, using a new tip for each reagent vessel.
6. Gently pour in remaining Abbott mSample Preparation bottles into the reagent vessels per the table above except for mMicroparticlesDNA which will be loaded later.
A total of 96 samples can be processed in each run. A negative control and a positive control are included in each run, therefore allowing a maximum of 94 specimens to be processed per run.
- If the Transport Tube contained within an Abbott multi-Collect Specimen Collection Kit is used for the storage and/or transport of specimens, or if a sample has been transferred to an Abbott Transport Tube (List No. 04J71-81), the minimum sample volume and associated rack requirements on the Abbott m2000sp are:

- For specimens in sample tubes other than the Abbott Transport Tube, the Abbott RealTime SARS-CoV-2 assay minimum sample volume and associated rack requirements on the Abbott m2000sp are:

- If frozen, thaw specimens at 15 to 30°C or at 2 to 8°C. Once thawed, specimens can be stored at 2 to 8°C for up to 6 hours if not processed immediately.
NOTE: For every stored specimen, if centrifugation is needed, the following actions must be done in the order described: vortex the specimen first and follow with centrifugation of respiratory specimens. If these actions are not performed in this order, then invalid results may occur.
- Vortex each specimen 3 times for 2 to 3 seconds.
- If needed, centrifuge respiratory specimens only at 2000 g for 5 minutes before loading onto the Abbott m2000sp worktable. Aliquot each specimen into clean tubes or vials if necessary. Refer to the Abbott m2000sp Operations Manual for tube sizes. Avoid touching the inside of the cap when opening tubes.
Refer to the Abbott m2000sp Operations Manual for tube sizes.
7. Remove cap. Avoid touching the inside of the cap when opening tubes. Remove swab if present.
8. Place the positive and negative controls, if applicable, and the patient specimens into the Abbott m2000sp sample rack. If used, bar codes on tube labels must face right for scanning.
9. Place the 5 mL Reaction Vessels into the Abbott m2000sp 1 mL subsystem carrier.
10. Immediately prior to initiation of the sample extraction protocol, vigorously mix or vortex the mMicroparticlesDNA until they are fully resuspended and pour the mMicroparticlesDNA into the appropriate 200 mL reagent vessel.
11. Load the Abbott mSample Preparation SystemDNA reagents and the Abbott 96 Deep-Well Plate on the Abbott m2000sp worktable as described in the Abbott m2000sp Operations Manual, Operating Instructions section.
12. From the Protocol screen, select the appropriate application file and initiate the sample extraction protocol as described in the Abbott m2000sp Operations Manual, Operating Instruction section.
- The application specification file m2000 SARS_CoV-2 is required for respiratory specimens.
- The Abbott m2000sp Master Mix Addition protocol (step 14) must be initiated within 1 hour after completion of Sample Preparation.
NOTE: Change gloves before handling the amplification reagents.
13. Load the amplification reagents and the master mix tube on the Abbott m2000sp worktable after sample preparation is completed.
The following table shows the number of amplification reagent packs needed based on the number of reactions.

- All amplification reagent packs used in runs of greater than 24 reactions must have the same lot number.
- Ensure that the contents of amplification reagent packs are at the bottom of the vials prior to opening the amplification reagents by tapping the vials in an upright position on the bench 5 to 10 times.
- Ensure that amplification reagent packs are firmly seated on the instrument.
14. Select the appropriate deep-well plate that matches the corresponding sample preparation extraction. Initiate the Abbott m2000sp Master Mix Addition protocol. Follow the instructions as described in the Abbott m2000sp Operations Manual, Operating Instructions section.
NOTE: The operator should not manually fill any empty/unfilled wells in the Abbott 96-Well Optical Reaction Plate.
- The Abbott m2000rt protocol (step 18) must be started within 50 minutes of the initiation of the Master Mix Addition protocol (step 14).
NOTE: If the run is aborted for any reason subsequent to step 14, a new 96-well PCR plate must be used if the Abbott m2000sp Master Mix Addition Protocol (step 14) will be repeated.
Amplification Area
15. Switch on and initialize the Abbott m2000rt instrument in the Amplification Area.
NOTE: The Abbott m2000rt requires 15 minutes to warm-up.
NOTE: Remove gloves before returning to the sample preparation area.
16. Seal the Abbott 96-Well Optical Reaction Plate according to the Abbott m2000sp Operations Manual, Operating Instructions section.
17. Place the sealed optical reaction plate into the Abbott Splash-Free Support Base for transfer to the Abbott m2000rt instrument. Export the completed PCR plate results to a CD (or directly to a mapped Abbott m2000rt via a network connection).
Abbott m2000rt Procedures
For a detailed description of how to perform the Abbott m2000rt SARS-CoV-2 assay protocol, refer to the Operating Instructions section in the Abbott m2000rt Operations Manual.
18. Place the Abbott 96-Well Optical Reaction Plate in the Abbott m2000rt instrument. Initiate the Abbott RealTime SARS-CoV-2 assay protocol (m2000 SARS-CoV-2), as described in the Abbott m2000rt Operations Manual, Operating Instructions section.
NOTE: Test order transfer through the use of CD-ROM or network connection with export and import features of the m2000sp and m2000rt software is recommended. If creating the Abbott m2000rt test order manually, enter sample IDs in the corresponding PCR tray locations according to the “Wells for Selected Plate” grid, found on the detail screen of the “PCR Plate Results” on the Abbott m2000sp. See Section 5 of the Abbott m2000sp Operations Manual.
POST PROCESSING PROCEDURES
- Remove the Abbott 96 Deep-Well Plate from the worktable and dispose of according to the Abbott m2000sp Operations Manual.
- Place the Abbott 96-Well Optical Reaction Plate in a sealable plastic bag and dispose according to the Abbott m2000rt Operations Manual along with the gloves used to handle the plate.
- Clean the Abbott Splash-Free Support Base before next use, according to the Abbott m2000rt Operations Manual.
QUALITY CONTROL PROCEDURES
Abbott m2000rt Optical Calibration
Refer to the Calibration Procedures section in the Abbott m2000rt Operations Manual for a detailed description of when and how to perform an Abbott m2000rt Optical Calibration.
Optical calibration of the Abbott m2000rt instrument is required for the accurate measurement and discrimination of dye fluorescence during the Abbott RealTime SARS-CoV-2 assay.
The following Abbott m2000rt Optical Calibration Plates are used to calibrate the Abbott m2000rt instrument for the Abbott RealTime SARS-CoV-2 assay:
- FAM™ Plate (Carboxyfluorescein)
- ROX™ Plate (Carboxy-X-rhodamine)
- VIC® Plate (Proprietary dye)
Detection of Inhibition
A defined, consistent quantity of IC nucleic acid is introduced into each specimen and control at the beginning of sample preparation and measured on the Abbott m2000rt to demonstrate proper specimen processing and assay validity. The IC is comprised of a RNA sequence unrelated to the SARS-CoV-2 virus target sequences. An IC CN validity range is defined within the Abbott RealTime SARS-CoV-2 Assay Application File.
An error code or flag is displayed when a specimen or control fails to meet the IC specification. Refer to INTERPRETATION OF RESULTS section of this package insert and the Abbott m2000rt System Operations Manual for a list of error codes and flags.
Negative and Positive Controls
A negative control and a positive control are included in each test order to evaluate run validity in order to generate a valid result.
The Abbott m2000rt instrument automatically reports the control results on the Abbott m2000rt workstation. An error control flag is displayed when a control result is out of range. Refer to the Abbott m2000rt Operations Manual for an explanation of the corrective actions for the error control flag. If negative or positive controls are out of range, all of the specimens and controls from that run must be reprocessed, beginning with sample preparation.
The presence of the SARS-CoV-2 virus must not be detected in the negative control. SARS-CoV-2 virus detected in the negative control is indicative of contamination by other samples or by amplified product introduced during sample preparation or during preparation of the Abbott 96-Well Optical Reaction Plate. To avoid contamination, clean the Abbott m2000sp instrument and the Abbott m2000rt instrument and repeat sample processing for controls and specimens following the Procedural Precautions. If negative controls are persistently reactive, contact your Abbott representative.
Monitoring the Laboratory for the Presence of Contamination
It is recommended that this test be done at least once a month to monitor laboratory surfaces and equipment for contamination by amplification product. It is very important to test all areas that may have been exposed to processed specimens, controls, and/or amplification product. This includes routinely handled objects such as pipettes, the Abbott m2000sp and Abbott m2000rt function keys, laboratory bench surfaces, microcentrifuges, and centrifuge adaptors.
- Add 0.8 mL RNase-free water to a 1.7 mL molecular biology grade microcentrifuge tube.
- Saturate the cotton tip of an applicator (Puritan or equivalent) in the RNase-free water from the microcentrifuge tube.
- Using the saturated cotton tip of the applicator, wipe the area to be monitored using a sweeping motion. Place the applicator into the microcentrifuge tube.
- Swirl the cotton tip in RNase-free water 10 times, and then press the applicator along the inside of the tube so that the liquid drains back into the solution at the bottom of the microcentrifuge tube. Discard the applicator.
- Pipette 0.5 mL of mWash 1 buffer to a clean tube using the pipette dedicated for Internal Control use.
- Add 20 μL of the mWash 1 buffer to each microcentrifuge tube.
- Cap the microcentrifuge tube.
- Test this sample according to the assay procedure section of this study brochure.
- Transfer liquid from the microcentrifuge tube to a 5 mL Reaction Vessel.
- Bring the volume to 1.5 mL with RNase-free water.
- The presence of contamination is indicated by the detection of SARS-CoV-2 nucleic acid in the swab samples.
- If SARS-CoV-2 nucleic acid is detected on equipment, follow the cleaning and decontaminating guidelines given in that equipment’s operations manual.
If SARS-CoV-2 nucleic acid is detected on surfaces, clean the contaminated areas with 1.0% (v/v) sodium hypochlorite solution, followed by 70% ethanol or water.
NOTE: Chlorine solutions may pit equipment and metal. Use sufficient amounts or repeated applications of 70% ethanol or water until chlorine residue is no longer visible. - Repeat testing of the contaminated area by following steps 1 through 10.
INTERPRETATION OF RESULTS
The Abbott m2000rt instrument automatically reports the results and interpretations on the Abbott m2000rt workstation. An error is displayed when a result is invalid. Assay results and interpretations will look similar to the following examples:

LIMITATIONS OF THE PROCEDURE
For use under an Emergency Use Authorization only.
- This assay is for in vitro diagnostic use under FDA Emergency Use Authorization only.
- Use of the Abbott RealTime SARS-CoV-2 assay is limited to personnel who have been trained in the procedures of a molecular diagnostic assay and the Abbott m2000 System.
- Laboratories are required to report all positive results to the appropriate public health authorities.
- The instruments and assay procedures reduce the risk of contamination by amplification product. However, nucleic acid contamination from the positive controls or specimens must be controlled by good laboratory practices and careful adherence to the procedures specified in this package insert.
- Optimal performance of this test requires appropriate specimen collection, storage, and transport to the test site (refer to the SPECIMEN COLLECTION, STORAGE, AND TRANSPORT TO THE TEST SITE section of this package insert).
- Detection of SARS-CoV-2 RNA may be affected by sample collection methods, patient factors (eg, presence of symptoms), and/or stage of infection.
- False-negative results may arise from degradation of the viral RNA during shipping/storage.
- The impacts of vaccines, antiviral therapeutics, antibiotics, chemotherapeutic or immunosuppressant drugs have not been evaluated.
- As with any molecular test, mutations within the target regions of Abbott RealTime SARS-CoV-2 assay could affect primer and/or probe binding resulting in failure to detect the presence of virus.
- Due to inherent differences between technologies, it is recommended that, prior to switching from one technology to the next, users perform method correlation studies in their laboratory to qualify technology differences. One hundred percent agreement between the results should not be expected due to aforementioned differences between technologies. Users should follow their own specific policies/procedures.
- Performance has only been established with the specimen types listed in the Intended Use. Other specimen types have not been evaluated and should not be used with this assay.
- Results should be interpreted by a trained professional in conjunction with the patient’s history and clinical signs and symptoms, and epidemiological risk factors.
- Negative results do not preclude infection with the SARS-CoV-2 virus and should not be the sole basis of a patient treatment/management or public health decision. Follow up testing should be performed according to the current CDC recommendations.
CONDITIONS OF AUTHORIZATION FOR LABORATORIES
The Abbott RealTime SARS-CoV-2 assay Letter of Authorization, along with the authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Patients, and authorized labeling are available on the FDA website: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas
However, to assist clinical laboratories using the Abbott RealTime SARS-CoV-2 assay (“your product” in the conditions below), the relevant Conditions of Authorization are listed below:
- Authorized laboratories1 using your product will include with result reports of your product, all authorized Fact Sheets. Under exigent circumstances, other appropriate methods for disseminating these Fact Sheets may be used, which may include mass media.
- Authorized laboratories using your product will use your product as outlined in the Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized extraction methods, authorized clinical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product will notify the relevant public health authorities of their intent to run your product prior to initiating testing.
- Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: [email protected]) and Abbott Molecular (email: [email protected]; 1-800-553-7042) any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
- All laboratory personnel using your product must be appropriately trained in RT-PCR techniques and use appropriate laboratory and personal protective equipment when handling this kit, and use your product in accordance with the authorized labeling.
1 The letter of authorization refers to, “laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform high complexity tests” as “authorized laboratories.”
SPECIFIC PERFORMANCE CHARACTERISTICS
Limit of Detection (Analytical Sensitivity)
Limit of Detection (LOD) studies determine the lowest detectable concentration of SARS-CoV-2 at which greater than or equal to 95% of all (true positive) replicates test positive.
To determine the LOD, a recombinant virus containing SARS-CoV-2 RNA (Seracare, AccuPlex COVID-19, 1.3E+07 copies/mL as determined by digital PCR) was serially diluted in simulated nasal matrix (SNM). The initial LOD was determined by testing 4 levels at target concentrations of 900, 300, 100, and 33 copies/mL. Each panel member was tested in replicates of 3. The final LOD was confirmed by testing 4 panel members with target concentrations at 400, 300, 200, and 100 copies/mL tested in replicates of 21.
The results are summarized in Table 1. The lowest concentration level with observed positive rates ≥ 95% was 100 virus copies/mL.

Inclusivity was demonstrated by analyzing the sequence of each of the SARS-CoV-2 primers and probes for homology with full-length SARS-CoV-2 sequences available in the GISAID database as of July 8, 2020 and in the NCBI database as of July 9, 2020.
Of the 60,577 SARS-CoV-2 strains evaluated, 59,588 (98.4%) exhibit 100% identity to all SARS-CoV-2 oligonucleotides. The remaining 989 sequences contain at least one mismatch in a SARS-CoV-2 oligonucleotide target region(s). 762 sequences contain at least one mismatch in an N oligonucleotide target region but show 100% identity in the associated RdRp oligonucleotide target regions. 205 sequences contain a single mismatch in an RdRp oligonucleotide target region but show 100% identity in the associated N oligonucleotide target regions. 22 sequences contain a single mismatch in an N oligo target region and a single mismatch in an RdRp oligo target region. Overall, the results of the analysis predict no impact to the detection of the 60,577 SARS-CoV-2 strains.
Cross-reactivity
Related pathogens, high prevalence disease agents and normal or pathogenic flora that are reasonably likely to be encountered in the clinical specimen have been evaluated in silico to identify the % homology between the selected probe/primer sequences and the sequence present in the microorganism.
The conclusion of this analysis is that there is limited opportunity for cross-reactivity to allow for false-positive reporting or affect performance of SARS-CoV-2 virus detection based upon the following:
- For many organisms, only one primer (forward or reverse) has >80% homology, making an amplified product unlikely.
- The probe is unlikely to bind for any of the hits (< 80% homology).
- Mismatches in the 3′ end of primers makes extension unlikely.
- For the N amplicon, two organisms with forward and reverse primers having >80% homology (LS483366.1, CP040804.1) have both primer binding sites on the same plus-sense strand and will not result in amplification.
- For the N amplicon, the remaining two organisms that may potentially give rise to amplicons due to both forward and reverse primers having >80% homology on opposite strands (CP000262.1, CP002888.1) have primer binding sites separated by >100,000 nucleotides in the bacterial chromosome, making amplification unlikely.
Overall, the results of this analysis predict no significant cross-reactivity or microbial interference.
Cross reactivity performance of Abbott RealTime SARS-CoV-2 assay was evaluated by testing whole organisms or appropriate representative samples listed below in Table 2.
No cross-reactivity of the Abbott RealTime SARS-CoV-2 assay with the selected microorganisms was observed at the concentrations tested.
The results are summarized in Table 2.


Clinical Performance Evaluation
A clinical evaluation study was performed to evaluate the performance of the Abbott RealTime SARS-CoV-2 Assay using nasopharyngeal swab specimens. A total of 61 contrived positive specimens at approximately 1X to 2X LOD and 20x LOD were tested. Samples were contrived by spiking known concentrations of recombinant virus containing SARS-CoV-2 RNA sequences into negative patient specimens. In addition to the contrived positive specimens, 34 negative specimens were tested.
There were 21 total samples tested at the 1X to 2X LOD level with 20 results valid and included in the analysis. One result was invalid and excluded from the analysis. There were 40 total samples tested at 20x LOD with 40 results valid and included in the analysis. There were 34 total samples tested for the negative level with 31 results valid and included in the analysis and 3 results invalid and excluded from the analysis.

An additional study evaluated the performance of the Abbott RealTime SARS-CoV-2 assay testing individual nasopharyngeal swab specimens (banked and acquired from a clinical lab). A total of 104 specimens were analyzed by both Abbott RealTime SARS-CoV-2 and Alinity m SARS-CoV-2 assays. Specimens acquired from the clinical lab were treated for viral inactivation at 65°C for 30 minutes prior to analysis. The positive percent agreement (PPA) between the 2 assays was 95.9% (47/49) and the negative percent agreement (NPA) was 100% (55/55). The results are summarized in Table 4.

BIBLIOGRAPHY
- US Department of Health and Human Services. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. Washington, DC: US Government Printing Office; December 2009. [Also available online. Type> www.cdc.gov, search>BMBL>look up sections III and IV.]
- US Department of Labor, Occupational Safety and Health Administration. 29 CFR Part 1910.1030. Bloodborne Pathogens.
- Clinical and Laboratory Standards Institute. Protection of Laboratory Workers from Occupationally Acquired Infections: Approved Guideline—Fourth Edition. CLSI Document M29-A4. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
- World Health Organization. Laboratory Biosafety Manual. 3rd ed. Geneva, Switzerland: World Health Organization; 2004.
- Clinical and Laboratory Standards Institute. Collection, Transport, Preparation, and Storage of Specimens for Molecular Methods; Approved Guideline. CLSI Document MM13-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2005.
- Centers for Disease Control and Prevention (CDC). Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19). Available online at: https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelinesclinical-
specimens.html

IN VITRO DIAGNOSTIC MEDICAL DEVICE
For technical assistance, call Abbott Molecular Technical Services at 1-800-553-7042, email [email protected], or visit the Abbott Molecular website at http://www.abbottmolecular.com.
Armored RNA® is a patented technology jointly developed by Ambion, Inc. and Cenetron Diagnostics, LLC. US patents #5,677,124, #5,919,625,#5,939,262 and patents pending.
Armored RNA is a registered trademark of Ambion. ProClin is a registered trademark of Rohm and Haas. FAM and ROX are trademarks of Life Technologies Corporation or its subsidiaries in the US and/or certain other countries. VIC is a registered trademark of Life Technologies Corporation or its subsidiaries in the US and/or certain other countries. Abbott m, m2000, m2000rt, and m2000sp are trademarks of Abbott Laboratories.
Abbott Molecular Inc. is the legal manufacturer of the:
Abbott RealTime SARS-CoV-2 Amplification Reagent Kit (List No. 09N77-095)
Abbott RealTime SARS-CoV-2 Control Kit (List No. 09N77-085)
Abbott RealTime SARS-CoV-2 Test Instructions – Optimized PDF
Abbott RealTime SARS-CoV-2 Test Instructions – Original PDF
References
]]>ABBOTT BinaxNOW Covid-19 Antigen Test Instructions
ABBOTT BinaxNOW Covid-19 Antigen Test Instructions
PROCEDURE CARD
For Use Under an Emergency Use Authorization (EUA) Only.
The BinaxNOW COVID-19 Ag Card is a lateral flow immunoassay for the qualitative detection of the nucleocapsid protein antigen to SARS-CoV-2 directly from anterior nasal (nares) swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within seven days of the onset of symptoms.
IMPORTANT: See Product Insert, including QC section, for complete use instructions, warnings, precautions and limitations.
False negative results may occur if specimens are tested past 1 hour of collection. Specimens should be tested as quickly as possible after specimen collection. Open the test card just prior to use, lay it flat, and perform assay as follows.
Part 1 – Sample Test Procedure
Patient Samples require 6 drops of Extraction Reagent
- Correct
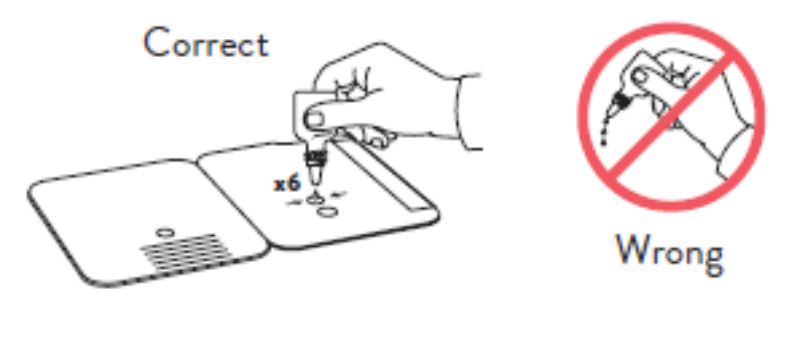
Hold Extraction Reagent bottle vertically. Hovering 1/2
inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE of the swab well. DO NOT touch the card
with the dropper tip while dispensing.
2.
Insert sample or control swab into BOTTOM HOLE and
firmly push upwards so that the swab tip is visible in the
TOP HOLE.
 3.
3.
Rotate (twirl) swab shaft 3 times CLOCKWISE (to
the right). Do not remove swab.
4.

Used test cards should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
Peel off adhesive liner from the right edge of the test card.
Close and securely seal the card. Read result in the window
15 minutes after closing the hard. In order to ensure proper
test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.
In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories; use by laboratories certifed under the CLIA, 42 U.S.C. §263a, that meet requirements to perform moderate, high or waived
complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certifcate of Waiver, Certifcate of Compliance, or Certifcate of Accreditation. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. In the USA, – this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of the virus that causes COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked
sooner.
Part 2 – Result Interpretation
Negative Result
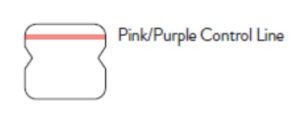
A negative specimen will give a single pink/purple colored Control
Line in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected. Negative results should be treated as presumptive and confirmation with a molecular
assay, if necessary, for patient management, may be performed.
Positive Result
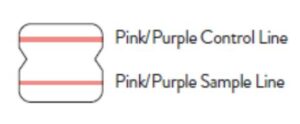
A positive specimen will give two pink/purple colored lines.
This means that COVID-19 antigen was detected. Specimens
with low levels of antigen may give a faint Sample Line. Any visible pink/purple colored line is positive.
Invalid Result
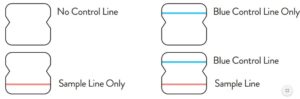
If no lines are seen, or if just the Sample Line is seen, the assay is
invalid. Invalid tests should be repeated.
Procedure for External Quality Control Testing
External Controls require 8 drops of Extraction ReagentHold
- Extraction Reagent bottle vertically. Hovering 1/2 inch
above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing. - Follow Steps 2 – 4 of the Test Procedure shown.
BinaxNOWTM COVID-19 Ag CARD
For Use Under an Emergency Use Authorization (EUA) Only
For use with anterior nasal (nares) swab specimens For in vitro Diagnostic Use Only Rx Only
INTENDED USE
The BinaxNOWTM COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal (nares) swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of
Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
The BinaxNOWTM COVID-19 Ag Card does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal (nares) swabs during the acute phase of infection. Positive
results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not
rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities.
Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed. Negative results do not rule out SARSCoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the
context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
The BinaxNOWTM COVID-19 Ag Card is intended for use by medical professionals or trained operators who are proficient in performing rapid lateral flow tests. BinaxNOWTM COVID-19 Ag
Card is only for use under the Food and Drug Administration’s Emergency Use Authorization.
SUMMARY AND EXPLANATION OF THE TEST
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARSCoV-2 is an enveloped, single-stranded RNA virus of the β genus. The virus can cause mild to
severe respiratory illness and has spread globally, including the United States.
BinaxNOWTM COVID-19 Ag Card is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media. The BinaxNOWTM COVID-19 Ag Card kit contains all components required to carry out an assay for SARS-CoV-2.
PRINCIPLES OF THE PROCEDURE
The BinaxNOWTM COVID-19 Ag Card is an immunochromatographic membrane assay that uses
highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens. SARS-CoV-2 specific antibodies and a control antibody are immobilized onto a
membrane support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of
a cardboard, book-shaped hinged test card. To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test results are
interpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple colored lines. Results should not be read after 30 minutes.
REAGENTS AND MATERIALS
Materials Provided
Test Cards (40): A cardboard, book-shaped hinged test card containing the test strip
Extraction Reagent (1): Bottle containing 7.5 mL of extraction reagent
Nasal Swabs (40): Sterile swabs for use with BinaxNOWTM COVID-19 Ag Card test
Positive Control Swab (1) : Non-infectious recombinant SARS-CoV-2 nucleocapsid antigen dried onto a swab
Negative Control Swab: The use of a sterile patient swab ensures appropriate negative results are obtained
Product Insert (1)
Procedure Card (1)
Materials Required but not Provided
Clock, timer or stopwatch
Materials Available as an Optional Accessory
Swab Transport Tube Accessory Pack
PRECAUTIONS
- For in vitro diagnostic use.
- This product has not been FDA cleared or approved; but has been authorized by FDA
under an EUA for use by laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform moderate, high or waived complexity tests and at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. - Federal Law restricts this device to sale by or on the order of a licensed practitioner (US only).
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens
- This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
- Laboratories within the United States and its territories are required to report all results to the appropriate public health laboratories.
- 7. Treat all specimens as potentially infectious. Follow universal precautions when handling samples, this kit and its contents.
- Proper sample collection, storage and transport are essential for correct results.
- Leave test card sealed in its foil pouch until just before use. Do not use if pouch is damaged or open.
- Do not use kit past its expiration date.
- Do not mix components from different kit lots.
- Do not reuse the used test card.
- Inadequate or inappropriate sample collection, storage, and transport may yield false test results.
- Do not store or test specimens in viral transport media, as it may result in false positive or false negative results.
- All components of this kit should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
- Solutions used to make the positive control swab are non-infectious. However, patient samples, controls, and test cards should be handled as though they could transmit disease. Observe established precautions against microbial hazards during use and disposal.
- Wear appropriate personal protection equipment and gloves when running each test and handling patient specimens. Change gloves between handling of specimens suspected of COVID-19.
- INVALID RESULTS can occur when an insufficient volume of extraction reagent is added to the test card. To ensure delivery of adequate volume, hold vial vertically, ½ inch above the swab well, and add drops slowly.
- False Negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
- Swabs in the kit are approved for use with BinaxNOWTM COVID-19 Ag Card. Do not use other swabs.
- The Extraction Reagent packaged in this kit contains saline, detergents and preservatives that will inactivate cells and virus particles. Samples eluted in this solution are not suitable for culture.
- Do not store the swab after specimen collection in the original paper packaging, if storage is needed use a plastic tube with cap.
STORAGE AND STABILITY
Store kit at 2-30°C. The BinaxNOWTM COVID-19 Ag Card kit is stable until the expiration date marked on the outer packaging and containers. Ensure all test components are at room temperature before use.
QUALITY CONTROL
BinaxNOWTM COVID-19 Ag Card has built-in procedural controls. For daily quality control, Abbott suggests that you record these controls for each test run.
Procedural Controls:
- The pink-to-purple line at the “Control” position is an internal procedural control. If the test flows and the reagents work, this line will always appear.
- The clearing of background color from the result window is a negative background control. The background color in the window should be light pink to white within 15 minutes. Background color should not hinder reading of the test.
External Positive and Negative Controls:
Good laboratory practice suggests the use of positive and negative controls to ensure that test reagents are working and that the test is correctly performed. BinaxNOWTM COVID-19 Ag Card
kits contain a Positive Control Swab and Sterile Swabs that can be used as a Negative Control Swab. These swabs will monitor the entire assay. Test these swabs once with each new shipment
received and once for each untrained operator. Further controls may be tested in order to conform with local, state and/or federal regulations, accrediting groups, or your lab’s standard Quality
Control procedures.
If the correct control results are not obtained, do not perform patient tests or report patient results. Contact Technical Support during normal business hours before testing patient specimens.
SPECIMEN COLLECTION AND HANDLING
Test specimens immediately after collection for optimal test performance. Inadequate specimen collection or improper sample handling/storage/transport may yield erroneous results. Refer to
the CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019 nCoV/lab/guidelines-clinical-specimens.html
Anterior Nasal (Nares) Swab
Only the swab provided in the kit is to be used for nasal swab collection.
To collect a nasal swab sample, carefully insert the entire absorbent tip of the swab (usually ½ to ¾ of an inch (1 to 1.5 cm) into the nostril. Firmly sample the nasal wall by rotating the swab in a circular path against the nasal wall 5 times or more for a total of
15 seconds, then slowly remove from the nostril. Using the same swab, repeat sample collection in the other nostril.
SPECIMEN TRANSPORT AND STORAGE
Do not return the nasal swab to the original paper packaging.
For best performance, direct nasal swabs should be tested as soon as possible after collection. If immediate testing is not possible, and to maintain best performance and avoid possible contamination, it is highly recommended the nasal swab is placed in a clean, unused plastic tube labeled with patient information, preserving sample integrity, and capped tightly at room temperature (15-30°C) for up to (1) hour prior to testing. Ensure the swab fits securely within the tube and the cap is tightly closed. If greater than 1 hour delay occurs, dispose of sample. A new sample must be collected for testing.
TEST PROCEDURE
Open the test card just prior to use, lay it flat, and perform assay as follows. The test card must be flat when performing testing, do not perform testing with the test card in any other position.
- Hold Extraction Reagent bottle vertically. Hovering 1/2 inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.

- Insert sample into BOTTOM HOLE and firmly push upwards so that the swab tip is visible in the TOP HOLE.

- Rotate (twirl) swab shaft 3 times CLOCKWISE (to the right). Do not remove swab.

Note: False negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
4. Peel off adhesive liner from the right edge of the test card. Close and securely seal the card. Read result in the window 15 minutes after closing the card. In order to ensure proper test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.

Note: False negative results can occur if test results are read before 15 minutes.
Note: When reading test results, tilt the card to reduce glare on the result window if necessary. Individuals with color-impaired vision may not be able to adequately interpret test results.
Procedure for BinaxNOW™ Swab Controls
Open the test card just prior to use, lay it flat, and perform assay as follows.
- Hold Extraction Reagent bottle vertically Hovering 1/2 inch above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.

- Follow Steps 2 – 4 of the Test Procedure for Patient Specimens.
RESULT INTERPRETATION
Note: In an untested BinaxNOW COVID-19 Ag Card there will be a blue line present at the Control Line position. In a valid, tested device, the blue line washes away and a pink/purple line appears, confirming that the sample has flowed through the test strip and the reagents are working. If the blue line is not present at the Control Line position prior to running the test, do not use and discard the test card.
Negative

A negative specimen will give a single pink/purple
colored Control Line in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected.
Positive

A positive specimen will give two pink/purple colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint Sample Line. Any visible pink/purple colored line is positive.
Invalid

If no lines are seen, if just the Sample Line is seen, or the Blue Control Line remains blue, the assay is invalid. Invalid tests should be repeated.
LIMITATIONS
- This test detects both viable (live) and non-viable, SARS-CoV, and SARS-CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- The performance of the BinaxNOW™ COVID-19 Ag Card was evaluated using the procedures provided in this product insert only. Modifications to these procedures may alter the
performance of the test. - False negative results may occur if a specimen is improperly collected, transported, or handled.
- False results may occur if specimens are tested past 1 hour of collection. Specimens should be test as quickly as possible after specimen collection.
- False negative results may occur if inadequate extraction buffer is used (e.g., <6 drops).
- False negative results may occur if specimen swabs are not twirled within the test card.
- False negative results may occur if swabs are stored in their paper sheath after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- False negative results are more likely after eight days or more of symptoms.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
- The presence of mupirocin may interfere with the BinaxNOW™ COVID-19 Ag test and may cause false negative results.
- Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed.
- If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
CONDITIONS of AUTHORIZATION for LABORATORY and PATIENT CARE SETTINGS
The BinaxNOW™ COVID-19 Ag Card Letter of Authorization, along with the authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Patients, and authorized labeling are available on the FDA website: https://www.fda.gov/medical devices/coronavirus-disease2019-covid-19-emergency-use- uthorizations-medical-devices/vitro-diagnostics-euas. However, to assist clinical laboratories using the BinaxNOW™ COVID-19 Ag Card, the relevant
Conditions of Authorization are listed below:
- Authorized laboratories1 using your product must include with test result reports, all authorized Fact Sheets. Under exigent circumstances, other appropriate methods for disseminating these Fact Sheets may be used, which may include mass media.
- Authorized laboratories using your product must use your product as outlined in the “BinaxNOW™ COVID-19 Ag Card” Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized clinical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product must notify the relevant public health authorities of their intent to run your product prior to initiating testing.
- Authorized laboratories using your product must have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: CDRH [email protected]) and Abbott Diagnostics Scarborough, Inc. (via email: [email protected], or via phone by contacting Abbott Diagnostics Scarborough, Inc. Technical Service at 1-800-257-9525) any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
- All operators using your product must be appropriately trained in performing and interpreting the results of your product, use appropriate personal protective equipment when handling this kit, and use your product in accordance with the authorized labeling.
- Abbott Diagnostics Scarborough, Inc., authorized distributors, and authorized laboratories using your product must ensure that any records associated with this EUA are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
1 The letter of authorization refers to, “Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform high, moderate, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.” as “authorized laboratories.”
PERFORMANCE CHARACTERISTICS
Clinical performance characteristics of BinaxNOW™ COVID-19 Ag Card was evaluated in a multisite prospective study in the U.S in which patients were sequentially enrolled and tested. A total
of ten (10) investigational sites throughout the U.S. participated in the study. Testing was performed by operators with no laboratory experience and who are representative of the intended users at CLIA waived testing sites. In this study testing was conducted by sixty-two (62) intended users. To be enrolled in the study, patients had to be presenting at the participating study centers with suspected COVID-19. Patients who presented within 7 days of symptom onset were included in the initial primary analysis. Two nasal swabs were collected from patients and tested using the
BinaxNOW™ COVID-19 Ag Card at all study sites. An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was utilized as the comparator method for this study. At all sites, one nasal swab was tested directly in the BinaxNOW™ COVID-19 Ag Card test according to product instructions and the other swab was eluted in viral transport media (VTM). Swabs were randomly assigned to testing with the BinaxNOW or RT-PCR testing and were tested
by minimally trained operators who were blinded to the RT-PCR test result. All sites shipped the VTM sample to a central testing laboratory for RT-PCR.
External control testing, using BinaxNOW™ COVID-19 Ag Card Positive and Negative Controls,
was performed prior to sample testing each day, at all study sites.
The performance of BinaxNOW™ COVID-19 Ag Card was established with 460 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW™ COVID-19 Ag Card Performance within 7 days of symptom onset against the Comparator Method.

*14 of the discrepant samples had high Ct values (>33) when tested by the comparator method.
The following data is provided for informational purposes:
The performance of BinaxNOW™ COVID-19 Ag Card with positive results stratified by the comparator method cycle threshold (Ct) counts were collected and assessed to better understand
the correlation of assay performance to the cycle threshold, estimating the viral titer present in the clinical sample. As presented in the table below, the positive agreement of the BinaxNOW™
COVID-19 Ag Card is higher with samples of a Ct count <33.
BinaxNOW™ COVID-19 Ag Card Performance against the Comparator Method – by Cycle Threshold Counts.

*In patients presenting within seven (7) days of symptom onset, BinaxNOW COVID-19 Ag Card achieved 95.6% (86/90) positive percent agreement for samples with Ct < 33
Patient Demographics
Patient demographics (gender and age) are available for the 460 samples used in the analysis of patients with symptom onset within the previous seven (7) days. The table below shows the positive results broken down by age of the patient:

Patient demographics, time elapsed since onset of symptoms for all patients enrolled, are presented in the table below. Positive results broken down by days since symptom onset:

A cohort of patients who presented with symptom onset greater than seven days were enrolled in the clinical study (n = 161). The positive agreement in patients with symptoms greater than seven
days was 60% (30/50) and negative agreement was 98% (109/111). Therefore, negative results in patients with symptom onset greater than seven days should be interpreted with caution, as the sensitivity of the assay decreases over time.
ANALYTICAL PERFORMANCE
BinaxNOW™ COVID-19 Ag Card limit of detection (LOD) was determined by evaluating different concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent. Inactivated SARS-CoV-2 virus was diluted in this
natural nasal swab matrix pool to generate virus dilutions for testing.
Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived swab samples were tested according to the test procedure.
The LOD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentration at which at least 19 out of 20 replicates tested positive).
The BinaxNOW™ COVID-19 Ag Card LOD in natural nasal swab matrix was confirmed as 140.6 TCID50/mL.
Limit of Detection (LoD) Study Results

Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW™ COVID-19 Ag Card was evaluated by testing 37 commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of heat inactivated SARS-CoV-2 virus
(45 TCID50/swab). No cross-reactivity or interference was seen with the following microorganisms when tested at the concentration presented in the table below.

To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the presence of organisms that were not available for wet testing, In silico analysis using the Basic Local Alignment Search
Tool (BLAST) managed by the National Center for Biotechnology information (NCBI) was used to assess the degree of protein sequence homology.
- For P. jirovecii one area of sequence similarity shows 45% homology across 18% of the sequence, making cross-reactivity in the BinaxNOW™ COVID-19 Ag Card highly unlikely.
- No protein sequence homology was found between M. tuberculosis, and thus homologybased cross-reactivity can be ruled out.
- The comparison between SARS-CoV-2 nucleocapsid protein, MERS-CoV and human coronavirus HKU1 revealed that cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of the sequence and 57.14% across 87% of the sequence, respectively
High Dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 1.6 x 105 TCID50/mL of heat inactivated SARS-CoV-2 virus with the BinaxNOW™ COVID-19 Ag Card.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the BinaxNOW™ COVID19 Ag Card at the concentrations listed below and were found not to affect test performance.

1-Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment: 20 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
ORDERING AND CONTACT INFORMATION
Reorder Numbers:
195-000: BinaxNOW™ COVID-19 Ag Card (40 Tests)
195-080: BinaxNOW™ COVID-19 Ag Control Swab Kit
US +1 877 441 7440
Technical Support Advice Line
Further information can be obtained from your distributor, or by contacting Technical Support on:
US
+ 1 800 257 9525
[email protected]
Abbott Diagnostics Scarborough, Inc. 10 Southgate Road Scarborough, Maine 04074 USA www.abbott.com/poct
FAQS
Abbott, not customers: what is the difference between this antigen self test and the binaxnow covid-19 ag card home test?
The BinaxNOW COVID-19 Antigen Self Test does not currently pair with the NAVICA app. The BinaxNOW COVID-19 Home Test can provide a sharable digital COVID-19 result, through collaboration with eMed. The BinaxNOW COVID-19 Antigen Self Test is the identical test card to the professional test but can be purchased at retail locations without a prescription. There’s also no need for a healthcare professional or online proctor to administer the swab or interpret results. Please let us know if there is anything else we can help you with.
Waiting for customer reviews. How accurate is the test?
This is an antigen test, not as accurate as a PCR molecular test. Depends on the viral load in your nasal passages. If positive you are likely infected with Covid. If negative and have symptoms test again and get a PCR test.
Can I use the BinaxNOW COVID-19 Ag Card for testing nasal swabs from contacts of confirmed SARS patients?
No. The BinaxNOW COVID-19 Ag Card is for testing only patients who are suspected of having SARS by their healthcare provider.
Can I use the BinaxNOW COVID-19 Ag Card to test nasal swabs from contacts of suspected SARS patients?
Yes. The BinaxNOW COVID-19 Ag Card is for testing nasal swabs from contacts of suspected SARS patients.
What if I have a negative result on the BinaxNOW COVID-19 Ag Card and my patient is still sick?
If your patient has a negative result on the BinaxNOW COVID-19 Ag Card and remains ill, contact your local or state health department immediately.
What if I have a positive result on the BinaxNOW COVID-19 Ag Card and my patient is not sick?
If your patient has a positive result on the BinaxNOW COVID-19 Ag Card and is not sick, contact your local or state health department immediately.
What if I have a positive result on the BinaxNOW COVID-19 Ag Card and my patient is not available for further testing?
If your patient has a positive result on the BinaxNOW COVID-19 Ag Card and is not available for further testing, contact your local or state health department immediately.
How long can I store the sample after collection before testing?
The sample must be tested as soon as possible after collection. Do not store samples longer than 24 hours at 2 to 8 degrees Celsius (36 to 46 degrees Fahrenheit). Do not freeze samples. After 24 hours, samples should be discarded.
What specific variants can this detect~ What variants were used in the efficacy test specimens? Are these specified in the package/materials?
Abbott is intently monitoring the mutations of COVID so we can ensure our tests can detect them, as we do with many viruses. We have conducted a thorough analysis of known variants that we’ve been able to study, and we are confident that our tests remain effective at identifying these strains. It is highly likely that future COVID-19 strains will remain detectable because our tests – and most other COVID-19 tests – look for parts of the virus that are crucial for the virus’ survival and therefore less subject to mutation. Please feel free to call 1-833-637-1594 if you need further assistance.
does it test for antibodies?
No, this is an antigen test that only detects an active infection. Antibody tests can detect past infections. Please let us know if there is anything else we can help you with.
What are the specificity and sensitivity ratings for this test?
Based on the interim results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
False positive and false negative rates for the test?
Based on the interim results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
Is this item hsa/fsa eligible?
That’s up to individual insurance companies to determine. Some cover other Over-the-Counter products so individuals would need to reach out to their provider to determine coverage.
Why did Abbott stop making this test?
We are producing millions of rapid tests per month here in the U.S. and, as we have been throughout the entire pandemic, are committed to making rapid tests available to more people at affordable prices and convenient places.
What is the QR code on the results card for?
The QR code on a BinaxNOW COVID-19 test card is used by NAVICA, Abbott’s COVID-19 digital testing system. NAVICA can be used to easily capture, manage and share self reported results from the BinaxNOW COVID-19 Antigen Self Test. It is available at no cost on the App Store or Google Play.
BinaxNOW Covid-19 Antigen Test Instructions – Optimized PDF
BinaxNOW Covid-19 Antigen Test Instructions – Original PDF
References
]]>This procedure is intended to provide a ready outline reference for performance of the assay. These abbreviated directions for use are not intended to replace the complete package insert.
Any modifications to this document are the sole responsibility of the Facility.
For Use Under an Emergency Use Authorization (EUA) Only For use with nasal swab specimens For in vitro Use Only Rx Only
1. Intended Use
The BinaxNOW™ COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS- CoV-2 in direct nasal swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
The BinaxNOW™ COVID-19 Ag Card does not differentiate between SARS- CoV and SARS-CoV-2. Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in nasal swabs during the acute phase of infection. Positive results indicate the presence
of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or coinfection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may
be performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions.
Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
The BinaxNOW™ COVID-19 Ag Card is intended for use by medical professionals or trained operators who are proficient in performing rapid lateral flow tests. BinaxNOW™ COVID-19 Ag Card is only for use under the Food and Drug Administration’s Emergency Use Authorization.
2. Summary and Explanation of the Test
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARS-CoV2 is an enveloped, single-stranded RNA virus of the β genus. The virus can cause mild to severe respiratory illness and has spread globally, including the United States.
BinaxNOW™ COVID-19 Ag Card is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media.
The BinaxNOW™ COVID-19 Ag Card kit contains all components required to carry out an assay for SARS-CoV-2.
3. Principles of the Procedure
The BinaxNOW™ COVID-19 Ag Card is an chromatography membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens.
SARS-CoV-2 specific antibodies and a control antibody are immobilized onto a membrane support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of a cardboard, book-shaped hinged test card.
To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is
inserted into the test card through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The swab is rotated 3 times clockwise and the
card is closed, bringing the extracted sample into contact with the test strip. Test results are interpreted visually at 15 minutes based on the presence or absence of visually detectable
pink/purple colored lines. Results should not be read after 30 minutes.
4. Reagents and Materials
Materials Provide
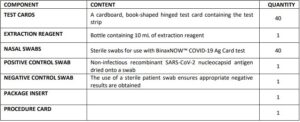
Materials Recommended But Not Provided
- Clock, timer or stopwatch
Materials Available as on Optional Accessory Item
- Swab Transport Tube Accessory Pack
5. Precautions
- For in vitro diagnostic use.
- This test has not been FDA cleared or approved; this test has been authorized by FDA under an EUA for use by laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform moderate, high, or waived complexity tests and at the Point of Care (POC), i.e., in patient care settings operating under a LIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- Federal Law restricts this device to sale by or on the order of a licensed practitioner (US only).
- This test has been authorized only for the detection of SARS-CoV-2 antigen, not for any other viruses or pathogens.
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
- Laboratories within the United States and its territories are required to report all positive results to the appropriate public health laboratories.
- Treat all specimens as potentially infectious. Follow universal precautions when handling samples, this kit and its contents.
- Proper sample collection, storage and transport are essential for correct results.
- Leave test card sealed in its foil pouch until just before use. Do not use if pouch is damaged or open.
- Do not use kit past its expiration date.
- Do not mix components from different kit lots.
- Do not reuse the used test card.
- Inadequate or inappropriate sample collection, storage, and ransport may yield false test results.
- Do not store specimens in viral transport media for specimen storage.
- All components of this kit should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
- Solutions used to make the positive control swab are non-infectious. However, patient samples, controls, and test cards should be handled as though they could transmit disease. Observe established precautions against microbial hazards during use and disposal.
- Wear appropriate personal protection equipment and gloves when running each test and handling patient specimens. Change gloves between handling of specimens suspected of COVID19.
- INVALID RESULTS can occur when an insufficient volume of extraction reagent is added to the test card. To ensure delivery of adequate volume, hold vial vertically, 1/2 inch above the swab well, and add drops slowly.
- False Negative results can occur if the sample swab is not rotated (twirled) prior to closing the ard.
- Swabs in the kit are approved for use with BinaxNOW™ COVID-19 Ag Card.Do not use other swabs.
- The Extraction Reagent packaged in this kit contains saline, detergents and preservatives thatwill inactivate cells and virus particles. Samples eluted in this solution are not suitable for culture.
- Do not store the swab after specimen collection in the original paper packaging, if storage is needed use a plastic tube with cap.
6. Storage and Stability
Store kit at 2-30°C. The BinaxNOW™ COVID-19 Ag Card kit is stable until the expiration date marked on the outer packaging and containers. Ensure all test components are at room temperature before use.
7. Quality Control
BinaxNOW™ COVID-19 Ag Card has built-in procedural controls. For daily quality control, Abbott suggests that you record these controls for each test run.
Procedural Controls:
A. The pink-to-purple line at the “Control” position is an internal procedural control. If the test
flows and the reagents work, this line will always appear.
B. The clearing of background color from the result window is a negative background control.
The background color in the window should be light pink to white within 15 minutes.
Background color should not hinder reading of the test.
External Positive and Negative Controls:
Good laboratory practice suggests the use of positive and negative controls to ensure that test reagents are working and that the test is correctly performed. BinaxNOW™ COVID-19 Ag Card kits
contain a Positive Control Swab and Sterile Swabs that can be used as a Negative Control Swab. These swabs will monitor the entire assay. Test these swabs once with each new shipment received
and once for each untrained operator. Further controls may be tested in order to conform with local, state and/or federal regulations, accrediting groups, or your lab’s standard Quality Control
procedures.
If the correct control results are not obtained, do not perform patient tests or report patient results.
Contact Technical Support during normal business hours before testing patient specimens.
8. Specimen Collection & Handling
Test specimens immediately after collection for optimal test performance. Inadequate specimen collection or improper sample handling/storage/ transport may yield erroneous results. Refer to the
CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019- nCoV/lab/guidelinesclinical-specimens.html Nasal Swab
Only the swab provided in the kit is to be used for nasal swab collection.To collect a nasal swab sample, carefully insert the swab into the nostril exhibiting the most visible drainage, or the nostril that is most congested if drainage is not visible. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril). Rotate the swab 5 times or more against the nasal wall then slowly remove from the nostril. Using the same swab, repeat sample collection in the other nostril.
9. Specimen Transport and Storage
Do not return the nasal swab to the original paper packaging.
For best performance, direct nasal swabs should be tested as soon as possible after collection. If immediate testing is not possible, and to maintain best performance and avoid possible contamination, it is highly recommended the nasal swab is placed in a clean, unused plastic tube labeled with patient information, preserving sample integrity, and capped tightly at room temperature (15-30°C) for up to (1) hour prior to testing. Ensure the swab fits securely within the
tube and the cap is tightly closed. If greater than 1-hour delay occurs, dispose of sample. A new sample must be collected for testing.
10. Test Procedure
Procedure for Patient Specimens
Open the test card just prior to use, lay it flat, and perform assay as follows. The test card must be flat when performing testing, do not perform testing with the test card in any other position.
- Hold Extraction Reagent bottle vertically. Hovering 1/2 inch
above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.
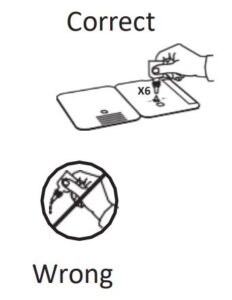
2. Insert sample into BOTTOM HOLE and firmly push upwards so
that the swab tip is visible in the TOP HOLE.
3. Rotate (twirl) swab shaft 3 times CLOCKWISE (to the right). Do
not remove swab.
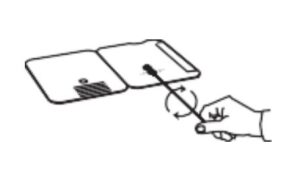
Note: False negative results can occur if the sample swab is not
rotated (twirled) prior to closing the card.
4. Peel off adhesive liner from the right edge of the test card.
Close and securely seal the card. Read result in the window 15
minutes after closing the card. In order to ensure proper test
performance, it is important to read the result promptly at 15
minutes, and not before. Results should not be read after 30
minutes.
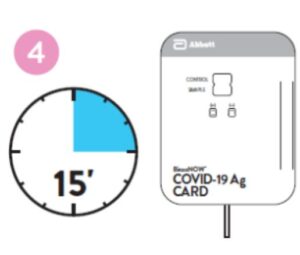
Note: When reading test results, tilt the card to reduce glare on
the result window if necessary. Individuals with color-impaired
vision may not be able to adequately interpret test results.
Procedure for BinaxNOW™ Swab Control
Open the test card just prior to use, lay it flat, and perform assay as follows.
- Hold Extraction Reagent bottle vertically Hovering 1/2 inch
above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.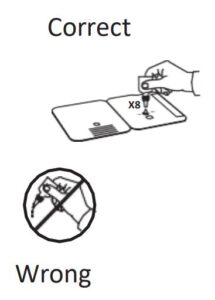
- Follow Steps 2 – 4 of the Test Procedure for Patient Specimens.
11. Result Interpretation
Note: In an untested BinaxNOW™ COVID-19 Ag Card there will be a blue line present at the Control Line position. In a valid, tested device, the blue line washes away and a pink/purple line appears, confirming that the sample has flowed through the test strip and the reagents are working. If the blue line is not present at the Control Line position prior to running the test, do not use and discard the test card.
Negative
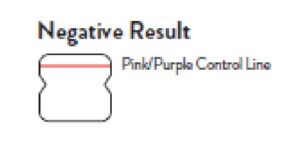
A negative specimen will give a single pink/purple colored
Control Line in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected.
Positive
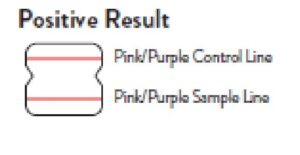
A positive specimen will give two pink/ purple colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint Sample Line. Any visible pink/ purple colored line is positive.
Invalid
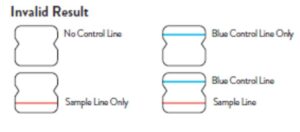
If no lines are seen, if just the Sample Line is seen, or the
Blue Control Line remains blue, the assay is invalid. Invalid
tests should be repeated.
12. Limitations
- • This test detects both viable (live) and non-viable, SARS-CoV and SARS- CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not
correlate with viral culture results performed on the same sample. - A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- The performance of the BinaxNOW™ COVID-19 Ag Card was evaluated using the procedures provided in this product insert only. Modifications to these procedures may alter the
performance of the test. - False negative results may occur if a specimen is improperly collected, transported, or handled.
- False results may occur if specimens are tested past 1 hour of collection. Specimens should be tested as quickly as possible after specimen collection.
- False negative results may occur if inadequate extraction buffer is used (e.g., <6 drops).
- False negative results may occur if specimen swabs are not wirled within the test card.
- False negative results may occur if swabs are stored in their paper sheath after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- Positive test results do not differentiate between SARS-CoV and SARS- CoV-2.
- Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
- The presence of mupirocin may interfere with the BinaxNOW™ COVID-19 Ag test and may cause false negative results.
- Negative results, from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management,
may be performed. - If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
Conditions of Authorization for Laboratory and Patient Care Settings
The BinaxNOW™ COVID-19 Ag Card Letter of Authorization, along with the authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Patients, and authorized labeling are available on the FDA website: https://www. fda.gov/medical-devices/coronavirus-disease-2019-covid-19- emergency-use- authorizations-medical-devices/vitro-diagnostics-euas.
However, to assist clinical laboratories using the BinaxNOW™ COVID-19 Ag Card, the relevant Conditions of Authorization are listed below:
- Authorized laboratories using your product will use your product as outlined in the “BinaxNOW™ COVID-19 Ag Card” Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized linical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product will notify the relevant public health authorities of their intent to run your product prior to initiating testing.
- Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: CDRH-EUA [email protected]) and Abbott Diagnostics Scarborough, Inc. (via email: [email protected], or via phone by contacting Abbott Diagnostics Scarborough, Inc. Technical Service at 1-800-257- 9525 any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware.
- All operators using your product must be appropriately trained in performing and interpreting the results of your product, use appropriate personal protective equipment when handling this
kit, and use your product in accordance with the authorized labeling. - Abbott Diagnostics Scarborough, Inc., authorized distributors, and authorized laboratories and patient care settings using your product will ensure that any records associated with this EUA
are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
1 The letter of authorization refers to, “Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform
high, moderate, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.” as “authorized laboratories.”
13. Performance Characteristics
Clinical Performance
Clinical performance characteristics of BinaxNOW™ COVID-19 Ag Card was evaluated in a multi-site prospective study in the U.S in which patients were sequentially enrolled and tested. A total of
seven (7) investigational sites throughout the U.S. participated in the study. Testing was performed by operators with no laboratory experience and who are representative of the intended users at
CLIA waived testing sites. In this study testing was conducted by thirty-two (32) intended users. No training on the use of the test was provided to the operators. To be enrolled in the study, patients
had to be presenting at the participating study centers with suspected COVID-19. Patients who presented within 7 days of symptom onset were included in the initial primary analysis, as only
seven asymptomatic patients were enrolled.
Of the seven asymptomatic patients, only two patients were positive for SARS-CoV-2. Two nasal swabs were collected from patients and tested using the BinaxNOW™ COVID-19 Ag Card at all study sites. An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was utilized as the comparator method for this study.
At all sites, one nasal swab was tested directly in the BinaxNOW™ COVID-19 Ag Card test according to product instructions and the other swab was eluted in viral transport media (VTM). Swabs were
randomly assigned to testing with the BinaxNOW™ or RT-PCR testing and were tested by minimally trained operators who were blinded to the RT-PCR test result. All sites shipped the VTM sample to a central testing laboratory for RT-PCR.
External control testing, using BinaxNOW™ COVID-19 Ag Card Positive and Negative Controls, was performed prior to sample testing each day, at all study sites.
The performance of BinaxNOW™ COVID-19 Ag Card was established with 102 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW™ COVID-19 Ag Card Performance against the Comparator Method
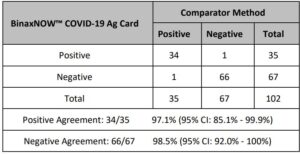
Patient Demographics
Patient demographics (gender, age, time elapsed since onset of symptoms) are available for the 102 samples used in the analysis. The table below shows the positive results broken down by age of the patient:
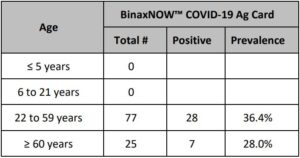
Positive results broken down by days since symptom onset:
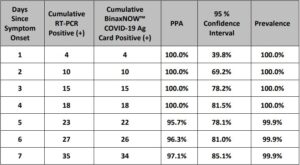
The following data is provided for informational purposes:
The performance of BinaxNOW™ COVID-19 Ag Card with positive results stratified by the comparator method cycle threshold (Ct) counts were collected and assessed to better understand
the correlation of assay performance to the cycle threshold, estimating the viral titer present in the clinical sample. As presented in the table below, the positive agreement of the BinaxNOW™ COVID19 Ag Card is higher with samples of a Ct count <33.
BinaxNOW™ COVID-19 Ag Card Performance against the Comparator Method – by Cycle Threshold Counts
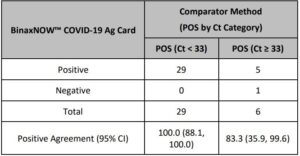
A limited cohort of patients who presented with symptom onset greater than seven days were enrolled in the clinical study (n = 28). Although the sample size was relatively small, the positive agreement in this cohort was 75% (9/12) and negative agreement was 92% (11/12). Therefore, negative results in patients with symptom onset greater than seven days should be treated as presumptive and confirmed with a molecular assay if needed for clinical management.
Analytical Performance:
Limit of Detection (Analytical Sensitivity)
BinaxNOW™ COVID-19 Ag Card limit of detection (LOD) was determined by evaluating different concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used
as the diluent. Inactivated SARS-CoV-2 virus was diluted in this natural nasal swab matrix pool to generate virus dilutions for testing.
Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived swab samples were tested according to the test procedure.
The LOD was determined as the lowest virus concentration that was detected ≥ 95% of the time (i.e., concentration at which at least 19 out of 20 replicates tested positive).
The BinaxNOW™ COVID-19 Ag Card LOD in natural nasal swab matrix was confirmed as 22.5 TCID50/swab.
Limit of Detection (LoD) Study Results

Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW™ COVID-19 Ag Card was evaluated by testing 37 commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of heat inactivated SARS-CoV-2 virus (45 TCID50/swab). No crossreactivity or interference was seen with the following microorganisms when tested at the concentration presented in the table below.
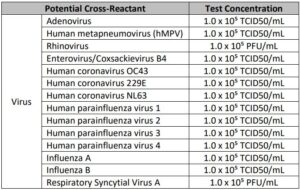
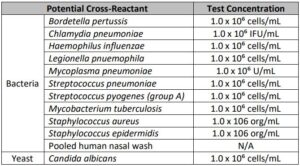
To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the presence of organisms that were not available for wet testing, In silico analysis using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for Biotechnology Information (NCBI) was used to assess the degree of protein sequence homology.
- For P. jirovecii one area of sequence similarity shows 45% homology across 18% of the sequence, making cross-reactivity in the BinaxNOW™ COVID-19 Ag Card highly unlikely.
- No protein sequence homology was found between M. tuberculosis, and thus homology-based cross-reactivity can be ruled out.
- The comparison between SARS-CoV-2 nucleocapsid protein, MERS-CoV and human coronavirus HKU1 revealed that cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of the sequence and 57.14% across 87% of the sequence, respectively.
High Dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 1.6 x 105 TCID50/mL of heat inactivated SARS-CoV-2 virus with the BinaxNOW™ COVID-19 Ag Card.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the BinaxNOW™ COVID-19 Ag Card at the concentrations listed below and were found not to affect test performance
1 Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment: 20 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
Ordering and Contact Information
Reorder numbers:
195-000: BinaxNOW™ COVID-19 Ag Card (40 Tests)
195-080: BinaxNOW™ COVID-19 Ag Control Swab Kit
190-010: Swab Transport Tube Accessory Pack
US + 1 877 441 7440
Technical Support Advice Line
Further information can be obtained by contacting Technical Support on:
US
+ 1 800 257 9525 [email protected]
Abbott Diagnostics Scarborough, Inc.
10 Southgate Road
Scarborough, Maine 04074 USA
www.globalpointofcare.abbott
Test Procedure Approval and Review Sheet
PREPARED BY:
DATE:
SUPERVISOR REVIEW:
DATE:
LABORATORY DIRECTOR OR
DESIGNEE APPROVAL:
IMPLEMENTATION DATE:
SUPERSEDES PROCEDURE
DATED:
DATE PROCEDURE RETIRED:
Method Comparison Study for BinaxNOW™ COVID-19 Ag Card
- Regulatory Background
The BinaxNOW™ COVID-19 Ag Card (195.000) does not require verification per the package insert/manufacturer recommendations, but some facilities may choose to complete.
This protocol should be followed as directed when performing a method comparison study of BinaxNOW™ COVID-19 Ag Card using patient samples. - Objective
The primary objective of this study is to estimate the positive percent agreement and negative percent agreement of the BinaxNOW™ COVID-19 Ag Card against the comparator method, in patients suspected of COVID-19 infection using healthcare worker-collected nasal swabs specimens tested directly (i.e. without dilution in viral transport media). - Study Population
Inclusion Criteria:
- Test direct nasal swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset.
- Select study sites with a good positivity rate and/or where clinicians are experienced in the diagnosis of COVID-19 (as good clinical sensitivity will increase the prevalence in the
sample of patients) so that the study can be completed in a timely fashion.
Exclusion Criteria:
- Do not try to find previously positive patients to quickly obtain positive samples.
- Subjects with active nose bleeds or acute facial injuries/trauma.
4. Sample Size
Generally, 20 samples (10 positive and 10 negative) are a minimum requirement1 , but low positivity rates can drive this number up. Please contact your inspecting agency (CAP, COLA, TJC,
or State) for current requirements. The depth of the verification study is determined by the laboratory director.
5. Recommendations on Study Design and Personnel Training
Study design and parameters should be established in order to ensure the proper training for healthcare staff on sample collection, handling, transport, and storage. Abbott Technical Consultants are available to assist with study design in addition to providing sample collection and end-user training (if facility staff is unable).
Two (2) patient swabs must be taken at the same time. One swab will be tested directly using the BinaxNOW™ COVID-19 Ag Card immediately after collection. The other swab will be tested
on the chosen comparator method. Depending on the manufacturer’s instructions, this swab will likely need to be put into transport media and transported to the lab. If verifications will be
conducted at multiple locations, the study design should be reviewed to gain insight from all participating sites.
During study design, it is important to consider the order of collection. For the best study comparison, nasal swabs should be tested by both the BinaxNOW™ COVID-19 Ag Card and the
comparator method. The study design should randomize the sequence of collection for each paired swab collected from a patient as assay performance can be impacted by unequal viral distribution due to differences in sample concentration.
Randomization of collection can ensure that this factor does not impact performance of the two methods. For instance, from Patient 1 the first paired swab should be tested on the BinaxNOW™
COVID-19 Ag Card and the second on the comparator method. For Patient 2 the first paired swab should be tested on the comparator method and the second on the BinaxNOW™ COVID-19
Ag Card.
These factors are critical components to ensure optimal study design. However, depending on specific situations other factors may also be critical to take into consideration. To ensure optimal
study design, Abbott Technical Consultants are available to assist.
6. Sample Collection, Handling, and Storage
As with any diagnostic test, the quality of sample collection, handling and storage are critical to assay performance. Freshly collected specimens should be used for optimal performance. Refer
to the CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-
nCoV/lab/guidelines-clinical-specimens.html. Two (2) paired swabs should be freshly collected in succession by a healthcare worker. The recommended procedures for collecting nasal swabs are provided below. Direct nasal should be tested as soon as possible after collection. If immediate testing is not possible the nasal swab
should be placed in a clean, unused plastic tube and capped tightly at room temperature (15- 30°C) for up to 1 hour prior to testing.
6.1 Nasal Swab Specimen Collection
With the first swab, use gentle rotation to push the swab into the right nostril until resistance is met at the level of the turbinates (less than one inch into the nostril). Rotate the swab five times
against the nasal wall then slowly remove from the nostril. Using the second swab, conduct sample collection in the left nostril.
With the second swab, use gentle rotation to push the swab into the right nostril until resistance is met at the level of the turbinates (less than one inch into the nostril). Rotate the 17 120007069 v04 12/20 BinaxNOW™ COVID-19 Ag Card swab five times against the nasal wall then slowly remove from the nostril. Using the first swab, repeat sample collection in the left nostril.
Cross over collection allows for equal loading of both swabs. Swab randomization accounts for difference in viral load between the left and right nostril.
Nasal Swab Specimen Collection2
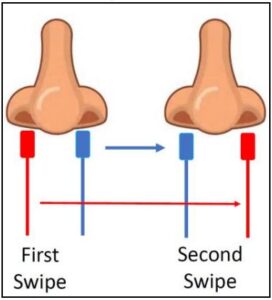
7. Comparator Testing
A comparator method should be selected for which study staff are trained and the same sample type can be evaluated as selected BinaxNOW™ COVID-19 Ag Card. As all products have specific
requirements, study design should consider the specific product package insert to optimize performance of both test methods. Quality and procedural controls should be followed as recommended by the specific product manufacturer and package insert.
8. Data Analysis
It is recommended that statistical analysis for positive percent agreement (PPA), negative percent agreement (NPA), and overall percent agreement are used to analyze and report study
data. An example of how to calculate and present the study results is shown below.
2 x 2 Contingency Table When Using a Comparative Method
BinaxNOW™
COVID-19 Ag Card
Comparative Method
Percent positive agreement (candidate method) = 100 x a / (a + c)
Percent negative agreement (candidate method) = 100 x d / (b + d)
Overall percent agreement = 100 x ( a + d) / n
9. BinaxNOW™ COVID-19 Ag Card Test Procedure4
Refer to the BinaxNOW™ COVID-19 Ag Card product insert.
If you have any questions or require further assistance on the BinaxNOW™ COVID-19 Ag Card, please contact Abbott Technical Support at: 800-257-9525 [email protected] 1CAP All Common Checklist 09172019 COM.40350 2Clinical Evaluation of the Investigational ID NOW™ COVID-19 Assay. Protocol Number: 2012601. Original (Version 1): 16 June 2020 Version 2: 01 July 2020 3CLSI EP12-A2:2008 User Protocol for Evaluation of Qualitative Test Performance, 2nd Edition. Jan 2008 Vol 28 (3). ISBN 1-56238-654-9 ISSN 0273-3099 4BinaxNOW™ COVID-19 Ag Card Part Number # 195.000 Test Kit Laboratory Procedure
BinaxNOW™ COVID-19 Ag Card Verification Form
ACCOUNT NAME:_________________________________
ADDRESS:______________________________________________________________________________________________________
TELEPHONE:_____________________________________
BINAXNOW™ COVID-19 Ag Card LOT /EXP:__________________________________________________
DATE:_________________________________
SUPERVISOR SIGNATURE:____________________________
Record the results from reference specimens below.
Record the Sample #, the BinaxNOW™ COVID-19 Ag Card results, Tester’s Initials, and any comments. After the BinaxNOW™ COVID-19 Ag Card results have been recorded (positive or negative) then record the Expected Results (positive or negative).

BinaxNOW™ COVID-19 Ag Card Verification Form (Continued)
REVIEW:___________________________ DATE:__________
LABORATORY DIRECTOR REVIEW AND APPROVAL FOR CLINICAL USE:____________________________
DATE:________________________
BinaxNOW™ COVID-19 Ag Card External Quality Control Log
Abbott recommends that external positive and negative controls be run:
-Once with each new shipment received
-Once for each untrained operator
-When required by local, state, and/or federal regulations, accrediting groups, or your lab’s Quality Control procedures
If the expected external control results are not obtained, do not report patient results. Contact Technical Service at 800-257-9525.
REVIEWED BY: ____________________ DATE: ____________________
BinaxNOW™ COVID-19 Ag Card Internal Controls and Patient Record
LOT NUMBER EXP._____________ DATE NAME OF______________ FACILITY________________________________
Record the Date, Patient’s Name, Patient Test Result, Internal Control Results and the tester’s initials.
Positive Internal Control = The pink-to-purple line at the “Control” position can be considered an internal positive procedural control.
Negative Internal Control = The background color in the window should be light pink to white within 15 minutes.
REVIEWED BY: ____________________ DATE: ____________________
Quality Assessment Review Form and Checklist
Corrective Action Form
TECHNOLOGIST:_____________________ DATE:_____________
SUPERVISOR:________________________ DATE:_____________
LABORATORY DIRECTOR:____________ DATE:_____________
Temperature Log
EQUIPMENT: _________________________
NAME OF FACILITY: _____________________
TO BE RECORDED AT THE BEGINNING OF EACH WORKDAY. TEMPERATURE RANGE: _______
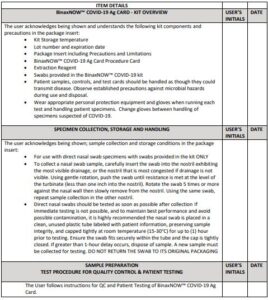
Tips for Successful Proficiency Testing (PT) Performance
- Strictly follow the PT provider’s storage or handling requirement before testing PT specimens.
- Analyze PT specimens within the time frame provided by the PT provider.
- Contact the PT provider promptly when specimens are received damaged. You may be able to receive a replacement immediately.
- Avoid clerical error when filling out PT answer sheets. Be sure to enter the correct result next to the correct analyte on the answer form.
- Remember to identify the instrument or method you are using to perform your PT so you are graded among your peer group.
- Make copies of all answer forms before submitting them to your PT provider.
- Please contact Technical Support at 800-257-9525 or [email protected] for further information on proficiency providers
BinaxNOW™ COVID-19 Ag Card – Training Checklist
FACILITY/LABORATORY:________________________________
USER NAME: ____________________________________________ USER ID:____________________
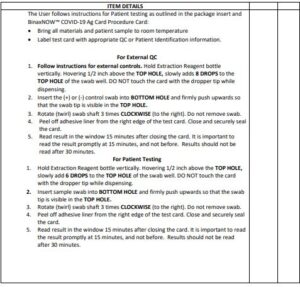
USER SIGNATURE_______________________________________________
DATE_____________________
TRAINER SIGNATURE______________________________________________
DATE ___________________
Certification of Training
This is to verify that personnel responsible for running the BinaxNOW™ COVID-19 Ag Card at
_____________________________ have been thoroughly in-serviced on the test and the test procedure.
This has included:
- Review of the package insert
- Demonstration of the product assay
- Successful performance of the BinaxNOW™ COVID-19 Ag Card and
interpretation of results
Names of the personnel who have been trained with the BinaxNOW™ COVID-19 Ag Card and are responsible for reporting patient results:
Signature of Laboratory Director(s) responsible for personnel and testing:
SIGNATURE_____________________ DATE:_________________
SIGNATURE_____________________ DATE:_________________
TRAINER_______________________ DATE:_____________
Testing Personnel Training Assessment
Test Method: BinaxNOW™ COVID-19 Ag Card

(Attach all supporting documents)
EVALUATOR:___________________DATE:________________
EMPLOYEE:_________________________________
BinaxNOW™ COVID-19 Ag Card Quiz
Name:__________________________________________
Date: _____________
Circle T (True) or F (False) for each Question:
- For testing patient direct nasal swabs, hold Extraction Reagent
bottle vertically Hovering 1/2 inch above the TOP HOLE, slowly
add 8 DROPS to the TOP HOLE of the swab well. - The BinaxNOW™ COVID-19 Ag Card device should not be
removed from the foil pouch until just before use. - A nasopharyngeal swab is an acceptable sample type for testing
on the BinaxNOW™ COVID-19 Ag Card. - Direct nasal swab samples may be stored at room temperature
for 24 hours. - It is acceptable to return the nasal swab after patient collection
to its original packaging. - False negative results can occur if the sample swab is not rotated
(twirled) prior to closing the card. - If no lines are seen, or if just the Sample Line is seen, the assay is
negative. - Results should be read promptly at 15 minutes, and not before.
Results should not be read after 30 minutes. - The appearance of a pink-to-purple Control Line and a pink-topurple Sample Line below it is a positive result.
- Test using only with swabs provided in the kit, and collect direct
nasal swabs from individuals suspected of COVID-19 by their
healthcare provider within the first seven days of symptom
onset.
BinaxNOW™ COVID-19 Ag Card Quiz Answer Key
- F For testing patient direct nasal swabs, hold Extraction Reagent bottle
vertically Hovering 1/2 inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE of the swab well. - T The BinaxNOW™ COVID-19 Ag Card device should be left in the foil pouch until just before use.
- F A direct nasal swab specimen is the only accepted sample type for the
BinaxNOW™ COVID-19 Ag Card kit. Other sample types are unacceptable. - F If immediate testing is not possible, the nasal swab must be returned to a clean, unused plastic tube labeled with patient information and can be held at room temperature (15- 30°C) for up to one (1) hour prior to testing.
- F Do not store the swab after specimen collection in the original paper
packaging, if storage is needed use a clean, unused plastic tube with cap. - T False negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
- F If no lines are seen, or if just the Sample Line is seen, the assay is invalid. Invalid tests should be repeated.
- T In order to ensure proper test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.
- T A positive BinaxNOW™ COVID-19 Ag Card will have a pink-to-purple control line and a second pink-to-purple sample line appears below it.
- T Test using only with swabs provided in the kit, and collect direct nasal swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset.
BinaxNOW COVID-19 Ag Card CLSI Lab Procedure [pdf]
BinaxNOW COVID-19 Ag Card CLSI Lab Procedure [doc]
References
]]>For Use Under an Emergency Use Authorization (EUA) Only
For use with nasal swab specimens
For in vitro Use Only

INTENDED USE
The BinaxNOW™ COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARSCoV-2 in direct nasal swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. The BinaxNOW COVID-19 Ag Card does not differentiate between SARSCoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in nasal swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
The BinaxNOW COVID-19 Ag Card is intended for use by medical
professionals or trained operators who are proficient in performing rapid lateral flow tests. BinaxNOW COVID-19 Ag Card is only for use under the Food and Drug Administration’s Emergency Use Authorization.
SUMMARY and EXPLANATION of the TEST
Coronaviruses are a large family of viruses which may cause illness in animals or humans. SARS-CoV-2 is an enveloped, single-stranded RNA virus of the β genus. The virus can cause mild to severe respiratory illness and has spread globally, including the United States.
BinaxNOW COVID-19 Ag Card is a rapid lateral flow immunoassay for the
qualitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media.
The BinaxNOW COVID-19 Ag Card kit contains all components required to
carry out an assay for SARS-CoV-2.
PRINCIPLES of the PROCEDURE
The BinaxNOW COVID-19 Ag Card is an immunochromatographic membrane assay that uses highly sensitive antibodies to detect SARS-CoV-2 nucleocapsid protein from nasal swab specimens. SARS-CoV-2 specific antibodies and a control antibody are immobilized onto a membrane support as two distinct lines and combined with other reagents/pads to construct a test strip. This test strip and a well to hold the swab specimen are mounted on opposite sides of a cardboard, book-shaped hinged test card.
To perform the test, a nasal swab specimen is collected from the patient, 6 drops of extraction reagent from a dropper bottle are added to the top hole of the swab well. The patient sample is inserted into the test card through the bottom hole of the swab well, and firmly pushed upwards until the swab tip is visible through the top hole. The swab is rotated 3 times clockwise and the card is closed, bringing the extracted sample into contact with the test strip. Test results are interpreted visually at 15 minutes based on the presence or absence of visually detectable pink/purple colored lines. Results should not be read after 30 minutes.
REAGENTS and MATERIALS
Materials Provided
Test Cards (40): A cardboard, book-shaped hinged test card containing the
test strip
Extraction Reagent (1): Bottle containing 10 mL of extraction reagent
Nasal Swabs (40): Sterile swabs for use with BinaxNOW COVID-19 Ag Card test
Positive Control Swab (1): Non-infectious recombinant SARS-CoV-2
nucleocapsid antigen dried onto a swab
Negative Control Swab: The use of a sterile patient swab ensures appropriate
negative results are obtained
Product Insert (1)
Procedure Card (1)
Materials Required but not Provided
Clock, timer or stopwatch
Materials Available as an Optional Accessory
Swab Transport Tube Accessory Pack
PRECAUTIONS
- For in vitro diagnostic use.
- This test has not been FDA cleared or approved; this test has been authorized by FDA under an EUA for use by laboratories certified under
the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42
U.S.C. §263a, to perform moderate, high, or waived complexity tests and at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. - Federal Law restricts this device to sale by or on the order of a licensed
practitioner (US only). - This test has been authorized only for the detection of SARS-CoV-2
antigen, not for any other viruses or pathogens. - This test is only authorized for the duration of the declaration that
circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. - Laboratories within the United States and its territories are required to
report all positive results to the appropriate public health laboratories. - Treat all specimens as potentially infectious. Follow universal precautions when handling samples, this kit and its contents.
- Proper sample collection, storage and transport are essential for correct
results. - Leave test card sealed in its foil pouch until just before use. Do not use if pouch is damaged or open.
- Do not use kit past its expiration date.
- Do not mix components from different kit lots.
- Do not reuse the used test card.
- Inadequate or inappropriate sample collection, storage, and transport may yield false test results.
- Do not store specimens in viral transport media for specimen storage.
- All components of this kit should be discarded as Biohazard waste according to Federal, State and local regulatory requirements.
- Solutions used to make the positive control swab are non-infectious .
However, patient samples, controls, and test cards should be handled as
though they could transmit disease. Observe established precautions against microbial hazards during use and disposal. - Wear appropriate personal protection equipment and gloves when running each test and handling patient specimens. Change gloves between handling of specimens suspected of COVID-19.
- INVALID RESULTS can occur when an insufficient volume of extraction reagent is added to the test card. To ensure delivery of adequate volume, hold vial vertically, 1/2 inch above the swab well, and add drops slowly.
- False Negative results can occur if the sample swab is not rotated (twirled) prior to closing the card.
Swabs in the kit are approved for use with BinaxNOW COVID-19 Ag Card. Do not use other swabs. - The Extraction Reagent packaged in this kit contains saline, detergents and preservatives that will inactivate cells and virus particles. Samples eluted in this solution are not suitable for culture.
- Do not store the swab after specimen collection in the original paper
packaging, if storage is needed use a plastic tube with cap.
STORAGE and STABILITY
Store kit at 2-30°C. The BinaxNOW COVID-19 Ag Card kit is stable until the expiration date marked on the outer packaging and containers. Ensure all test components are at room temperature before use.
QUALITY CONTROL
BinaxNOW COVID-19 Ag Card has built-in procedural controls. For daily
quality control, Abbott suggests that you record these controls for each test run. Procedural Controls:
A. The pink-to-purple line at the “Control” position is an internal procedural control. If the test flows and the reagents work, this line will always appear.
B. The clearing of background color from the result window is a negative
background control. The background color in the window should be light
pink to white within 15 minutes. Background color should not hinder
reading of the test. External Positive and Negative Controls:
Good laboratory practice suggests the use of positive and negative controls to ensure that test reagents are working and that the test is correctly performed. BinaxNOW COVID-19 Ag Card kits contain a Positive Control Swab and Sterile Swabs that can be used as a Negative Control Swab. These swabs will monitor the entire assay. Test these swabs once with each new shipment received and once for each untrained operator. Further controls may be tested in order to conform with local, state and/or federal regulations, accrediting groups, or your lab’s standard Quality Control procedures.
If the correct control results are not obtained, do not perform patient tests or
report patient results. Contact Technical Support during normal business hours before testing patient specimens.
SPECIMEN COLLECTION and HANDLING
Test specimens immediately after collection for optimal test performance.
Inadequate specimen collection or improper sample handling/storage/
transport may yield erroneous results. Refer to the CDC Interim Guidelines
for Collecting, Handling, and Testing Clinical Specimens from Persons for
Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-
nCoV/lab/guidelines-clinical-specimens.html
Nasal Swab
Only the swab provided in the kit is to be used for nasal swab collection.
To collect a nasal swab sample, carefully insert the swab into the nostril exhibiting the most visible drainage, or the nostril that is most congested if drainage is not visible. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril). Rotate the swab 5 times or more against the nasal wall then slowly remove from the nostril. Using the same swab, repeat sample collection in the other nostril.
SPECIMEN TRANSPORT and STORAGE
Do not return the nasal swab to the original paper packaging.
For best performance, direct nasal swabs should be tested as soon as possible
after collection. If immediate testing is not possible, and to maintain best
performance and avoid possible contamination, it is highly recommended
the nasal swab is placed in a clean, unused plastic tube labeled with patient
information, preserving sample integrity, and capped tightly at room
temperature (15-30°C) for up to (1) hour prior to testing. Ensure the swab fits securely within the tube and the cap is tightly closed. If greater than 1 hour delay occurs, dispose of sample. A new sample must be collected for testing.
TEST PROCEDURE
Procedure for Patient Specimens
Open the test card just prior to use, lay it flat, and perform assay as follows.
The test card must be flat when performing testing, do not perform testing with the test card in any other position.
- Hold Extraction Reagent bottle vertically. Hovering 1/2 inch above the TOP HOLE, slowly add 6 DROPS to the TOP HOLE
of the swab well. DO NOT touch the card with the dropper tip while dispensing.
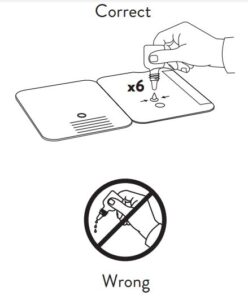
- Insert sample into BOTTOM HOLE and firmly push upwards so that the swab tip is visible in the TOP HOLE.
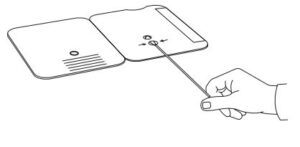
- Rotate (twirl) swab shaft 3 times
CLOCKWISE (to the right). Do not
remove swab.
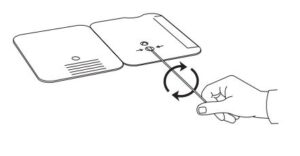
Note: False negative results can occur if the sample swab is not rotated (twirled) prior to closing the card. - Peel off adhesive liner from the right edge of the test card. Close and securely seal the card. Read result in the window 15 minutes after closing the card. In order to ensure proper test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.
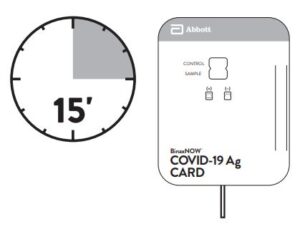
Note: When reading test results, tilt the card to reduce glare on the result window if necessary. Individuals with color-impaired vision may not be able to adequately interpret test results.
Procedure for BinaxNOW™ Swab Controls
Open the test card just prior to use, lay it flat, and perform assay as follows.
- Hold Extraction Reagent bottle vertically Hovering 1/2 inch above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.
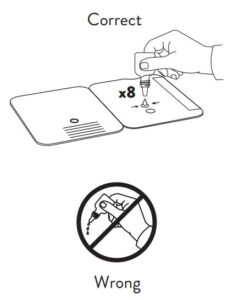
- Follow Steps 2 – 4 of the Test Procedure for Patient Specimens.
RESULT INTERPRETATION
Note: In an untested BinaxNOW COVID-19 Ag Card there will be a blue line present at the Control Line position. In a valid, tested device, the blue line washes away and a pink/purple line appears, confirming that the sample has flowed through the test strip and the reagents are working. If the blue line is not present at the Control Line position prior to running the test, do not use and discard the test card.
Negative

A negative specimen will give a single pink/purple colored Control Line in
the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected.
Positive

A positive specimen will give two pink/ purple colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint Sample Line. Any visible pink/ purple colored line is positive.
Invalid
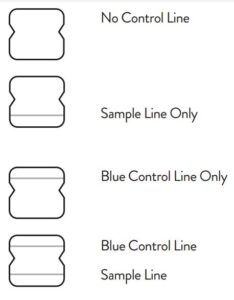
If no lines are seen, if just the Sample Line is seen, or the Blue Control Line
remains blue, the assay is invalid. Invalid tests should be repeated.
LIMITATIONS
- This test detects both viable (live) and non-viable, SARS-CoV and SARSCoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- The performance of the BinaxNOW COVID-19 Ag Card was evaluated
using the procedures provided in this product insert only. Modifications to these procedures may alter the performance of the test. - False negative results may occur if a specimen is improperly collected, transported, or handled.
- False results may occur if specimens are tested past 1 hour of collection.
Specimens should be tested as quickly as possible after specimen collection. - False negative results may occur if inadequate extraction buffer is used (e.g., <6 drops).
- False negative results may occur if specimen swabs are not twirled within the test card.
- False negative results may occur if swabs are stored in their paper sheath after specimen collection.
- Positive test results do not rule out co-infections with other pathogens.
- Positive test results do not differentiate between SARS-CoV and SARSCoV-2.
- Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
- The presence of mupirocin may interfere with the BinaxNOW COVID 19 Ag test and may cause false negative results.
- Negative results, from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed.
- If the differentiation of specific SARS viruses and strains is needed, additional testing, in consultation with state or local public health departments, is required.
CONDITIONS of AUTHORIZATION for
The BinaxNOW COVID-19 Ag Card Letter of Authorization, along with the
authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for
Patients, and authorized labeling are available on the FDA website: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-useauthorizations-medical-devices/vitro-diagnostics-euas.
However, to assist clinical laboratories using the BinaxNOW COVID-19 Ag
Card, the relevant Conditions of Authorization are listed below:
- Authorized laboratories1 using your product will include with test result
reports, all authorized Fact Sheets. Under exigent circumstances, other
appropriate methods for disseminating these Fact Sheets may be used, which may include mass media. - Authorized laboratories using your product will use your product as outlined in the “BinaxNOW COVID-19 Ag Card” Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized clinical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product will notify the relevant public health authorities of their intent to run your product prior to initiating testing.
- Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/CDRH (via email: [email protected]) and Abbott Diagnostics
Scarborough, Inc. (via email: [email protected], or via phone by contacting Abbott Diagnostics Scarborough, Inc. Technical Service at 1-800-257- 9525 any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of your product of which they become aware. - All operators using your product must be appropriately trained in performing and interpreting the results of your product, use appropriate personal protective equipment when handling this kit, and use your product in accordance with the authorized labeling
- Abbott Diagnostics Scarborough, Inc., authorized distributors, and authorized laboratories and patient care settings using your product will ensure that any records associated with this EUA are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
1 The letter of authorization refers to, “Laboratories certified under the Clinical
Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a,
that meet the requirements to perform high, moderate, or waived complexity
tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.” as “authorized laboratories.”
PERFORMANCE CHARACTERISTICS
CLINICAL PERFORMANCE
Clinical performance characteristics of BinaxNOW COVID-19 Ag Card
was evaluated in a multi-site prospective study in the U.S in which patients
were sequentially enrolled and tested. A total of seven (7) investigational
sites throughout the U.S. participated in the study. Testing was performed
by operators with no laboratory experience and who are representative of
the intended users at CLIA waived testing sites. In this study testing was
conducted by thirty-two (32) intended users. No training on the use of the
test was provided to the operators. To be enrolled in the study, patients had to
be presenting at the participating study centers with suspected COVID-19.
Patients who presented within 7 days of symptom onset were included in the
initial primary analysis, as only seven asymptomatic patients were enrolled.
Of the seven asymptomatic patients, only two patients were positive for
SARS-CoV-2. Two nasal swabs were collected from patients and tested using the BinaxNOW COVID-19 Ag Card at all study sites. An FDA Emergency Use Authorized real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was utilized as the comparator method for this study. At all sites, one nasal swab was tested directly in the BinaxNOW COVID-19 Ag Card test according to product instructions and the other swab was eluted in viral transport media (VTM). Swabs were randomly assigned to testing with the BinaxNOW or RT-PCR testing and were tested by minimally trained operators who were blinded to the RT-PCR test result. All sites shipped the VTM sample to a central testing laboratory for RT-PCR. External control testing, using BinaxNOW COVID-19 Ag Card Positive and Negative Controls, was performed prior to sample testing each day, at all study sites. The performance of BinaxNOW COVID-19 Ag Card was established with 102 nasal swabs collected from individual symptomatic patients (within 7 days of onset) who were suspected of COVID-19.
BinaxNOW™ COVID-19 Ag Card Performance within 7 days of symptom onset against the Comparator Method.
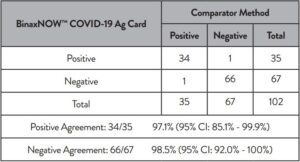
Patient Demographics
Patient demographics (gender, age, time elapsed since onset of symptoms) are available for the 102 samples used in the analysis. The table below shows the positive results broken down by age of the patient:
Positive results broken down by days since symptom onset:
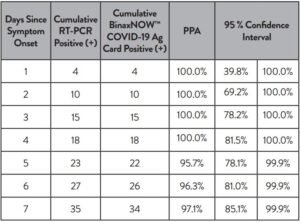
The following data is provided for informational purposes:
The performance of BinaxNOW COVID-19 Ag Card with positive results stratified by the comparator method cycle threshold (Ct) counts were collected and assessed to better understand the correlation of assay performance to the cycle threshold, estimating the viral titer present in the clinical sample. As presented in the table below, the positive agreement of the BinaxNOW COVID-19 Ag Card is higher with samples of a Ct count <33.
BinaxNOW™ COVID-19 Ag Card Performance against the Comparator Method – by Cycle Threshold Counts.
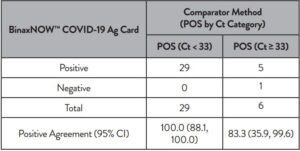
A limited cohort of patients who presented with symptom onset greater than
seven days were enrolled in the clinical study (n = 28). Although the sample size was relatively small, the positive agreement in this cohort was 75% (9/12) and negative agreement was 92% (11/12). Therefore, negative results in patients with symptom onset greater than seven days should be treated as presumptive and confirmed with a molecular assay if needed for clinical management.
ANALYTICAL PERFORMANCE:
Limit of Detection (Analytical Sensitivity)
BinaxNOW COVID-19 Ag Card limit of detection (LOD) was determined
by evaluating different concentrations of heat inactivated SARS-CoV-2 virus. Presumed negative natural nasal swab specimens were eluted in PBS. Swab eluates were combined and mixed thoroughly to create a clinical matrix pool to be used as the diluent. Inactivated SARS-CoV-2 virus was diluted in this natural nasal swab matrix pool to generate virus dilutions for testing. Contrived nasal swab samples were prepared by absorbing 20 microliters of each virus dilution onto the swab. The contrived swab samples were tested according to the test procedure.
The LOD was determined as the lowest virus concentration that was detected
≥ 95% of the time (i.e., concentration at which at least 19 out of 20 replicates
tested positive).
The BinaxNOW COVID-19 Ag Card LOD in natural nasal swab matrix was
confirmed as 22.5 TCID50/swab.
Limit of Detection (LoD) Study Results.

Cross Reactivity (Analytical Specificity) and Microbial Interference
Cross reactivity and potential interference of BinaxNOW COVID-19 Ag
Card was evaluated by testing 37 commensal and pathogenic microorganisms (8 bacteria, 14 viruses, 1 yeast and pooled human nasal wash) that may be present in the nasal cavity. Each of the organism, viruses, and yeast were tested in triplicate in the absence or presence of heat inactivated SARS-CoV-2 virus (45 TCID50/swab). No cross-reactivity or interference was seen with the following microorganisms when tested at the concentration presented in the table below.
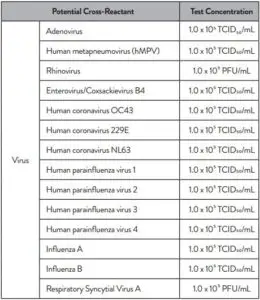
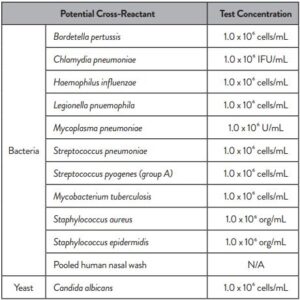
To estimate the likelihood of cross-reactivity with SARS-CoV-2 virus in the
presence of organisms that were not available for wet testing, In silico analysis using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for Biotechnology Information (NCBI) was used to assess the degree of protein sequence homology.
- For P. jirovecii one area of sequence similarity shows 45% homology
across 18% of the sequence, making cross-reactivity in the BinaxNOW COVID-19 Ag Card highly unlikely. - No protein sequence homology was found between M. tuberculosis, and thus homology-based cross-reactivity can be ruled out.
- The comparison between SARS-CoV-2 nucleocapsid protein, MERS CoV and human coronavirus HKU1 revealed that cross-reactivity cannot be ruled out. Homology for KHU1 and MERS-CoV is relatively low, at 37.8% across 95% of the sequence and 57.14% across 87% of the sequence, respectively.
High Dose Hook Effect
No high dose hook effect was observed when tested with up to a concentration of 1.6 x 105 TCID50/mL of heat inactivated SARS-CoV-2 virus with the BinaxNOW COVID-19 Ag Card.
Endogenous Interfering Substances
The following substances, naturally present in respiratory specimens or that may be artificially introduced into the nasal cavity or nasopharynx, were evaluated with the BinaxNOW COVID-19 Ag Card at the concentrations listed below and were found not to affect test performance.
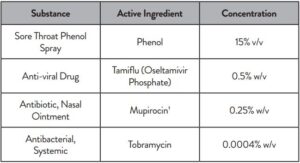
1 Testing demonstrated false negative results at concentrations of 5 mg/mL (0.5% w/v). Standard dose of nasal ointment: 20 mg (2% w/w) of mupirocin in single-use 1-gram tubes.
SYMBOLS

ORDERING and CONTACT INFORMATION
Reorder Numbers:
195-000: BinaxNOW COVID-19 Ag Card (40 Tests)
195-080: BinaxNOW COVID-19 Ag Control Swab Kit
190-010: Swab Transport Tube Accessory Pack
US +1 877 441 7440
Technical Support Advice Line
Further information can be obtained from your distributor, or by contacting
Technical Support on:
US
+ 1 800 257 9525 [email protected]

BinaxNOW COVID-19 Ag Card Package Insert – Optimized PDF
BinaxNOW COVID-19 Ag Card Package Insert – Original PDF
References
]]>
Abbott Diagnostics Scarborough, Inc. August 26, 2020
BinaxNOW™ COVID-19 Ag Card
This Fact Sheet informs you of the significant known and potential risks and benefits of the emergency use of the BinaxNOW COVID-19 Ag Card. The BinaxNOW COVID-19 Ag Card is authorized for use using nasal swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms.
All patients whose specimens are tested with this assay will receive the Fact Sheet for Patients: Abbott Diagnostics Scarborough, Inc. – BinaxNOW COVID-19 Ag Card.
What are the symptoms of COVID-19?
Many patients with confirmed COVID-19 have developed fever and/or symptoms of acute respiratory illness (e.g., cough, dyspnea). The current information available to characterize
the spectrum of clinical illness associated with COVID-19 suggests that symptoms include cough, shortness of breath or dyspnea, fever, chills, myalgias, headache, sore throat or
new loss of taste or smell, nausea or vomiting or diarrhea. Signs and symptoms may appear any time from 2 to 14 days after exposure to the virus, and the median time to symptom
onset is approximately 5 days. For further information on the symptoms of COVID-19 please see the link provided in “Where can I go for updates and more information?” section.
Public health officials have identified cases of COVID-19 infection throughout the world, including the United States. Please check the CDC COVID-19 webpage (see link provided in “Where can I go for updates and more information?” section at the end of this document) or your local jurisdictions website for the most up to date information.
What do I need to know about COVID-19 testing?
Current information on COVID-19 for healthcare providers is available at CDC’s webpage, Information for Healthcare Professionals (see links provided in “Where can I go for
updates and more information?” section).
- The BinaxNOW COVID-19 Ag Card can be used to test nasal swab samples directly using a dual nares collection (swab inserted in both nares).
- The BinaxNOW COVID-19 Ag Card should be ordered for the detection of COVID-19 in individuals who are suspected of COVID-19 by their healthcare provider and
who are within the first seven days of onset of symptoms. - The BinaxNOW COVID-19 Ag Card is only authorized for use in laboratories in the United States, certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform moderate, high and waived complexity tests. This test is authorized for use at the point of care (POC), i.e., in patient care settings operating under a CLIA certificate of Waiver, certificate of compliance, or certificate of accreditation.
This test is to be performed only using nasal swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms.
Specimens should be collected with appropriate infection control precautions. Current guidance for COVID-19 infection control precautions are available at the CDC’s website (see links provided in “Where can I go for updates and more information?” section). When collecting and handling specimens from individuals suspected of being infected with COVID-19, appropriate personal protective equipment should be used as outlined in the CDC Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19). For additional information, refer to CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19) (see links provided in “Where can I go for updates and more information?” section).
What does it mean if the specimen tests positive for the virus that causes COVID-19?
A positive test result for COVID-19 indicates that antigens from SARS-CoV-2 were detected, and the patient is infected with the virus and presumed to be contagious. Laboratory test results should always be considered in the context of clinical observations and epidemiological data in making a final diagnosis and patient management decisions. Patient management should follow current CDC guidelines. The BinaxNOW COVID-19 Ag Card has been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks to patients could include the following: a recommendation for isolation of the patient, monitoring of household or other close contacts for symptoms, patient isolation that might limit contact with family or friends and may increase contact with other potentially COVID-19 patients, limits in the ability to work, the delayed diagnosis and treatment for the true infection causing the symptoms, unnecessary prescription of a treatment or therapy, or other unintended adverse effects. All laboratories using this test must follow the standard testing and reporting guidelines according to their appropriate public health authorities.
What does it mean if the specimen tests negative for the virus that causes COVID-19?
A negative test result for this test means that antigens from SARS-CoV-2 were not present in the specimen above the limit of detection. However, a negative result does not rule
out COVID-19 and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Antigen tests are known to be less sensitive than molecular tests that detect viral nucleic acids. The amount of antigen in a sample may decrease as the duration of illness increases. Specimens collected after day 7 of illness
may be more likely to be negative compared to a RT-PCR assay. Therefore, negative results from patients with symptom onset beyond 7 days should be treated as presumptive and
confirmed with a molecular assay, if necessary, for patient management.
When diagnostic testing is negative, the possibility of a false negative result should be considered in the context of a patient’s recent exposures and the presence of clinical signs
and symptoms consistent with COVID-19. The possibility of a false negative result should especially be considered if the patient’s recent exposures or clinical presentation indicate that COVID-19 is likely, and diagnostic tests for other causes of illness (e.g., other respiratory illness) are negative.
If COVID-19 is still suspected based on exposure history together with other clinical findings, re-testing or testing with molecular methods should be considered by healthcare
providers in consultation with public health authorities. Risks from a false negative result include: delay or lack of supportive treatment, lack of monitoring of infected individuals
and their household or other close contacts for symptoms resulting in increased risk of spread of COVID-19 within the community, or other unintended adverse events. A negative antigen test should not be the sole basis used to determine if a patient can end isolation precautions. For additional recommendations regarding infection control, refer to
CDC’s Discontinuation of Isolation for Persons with COVID-19 Not in Healthcare Settings (Interim Guidance) (see links provided in “Where can I go for updates and more information” section).
What is an EUA?
The United States FDA has made this test available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA is supported by the Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the emergency use of in vitro diagnostics (IVDs) for the detection and/or diagnosis of the virus that causes COVID-19. An IVD made available under an EUA has not undergone the same type of review as an FDA-approved or cleared IVD. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, available alternatives, and based on the totality of scientific evidence available, it is reasonable to believe that this IVD may be effective in diagnosing COVID-19.
The EUA for this test is in effect for the duration of the COVID-19 declaration justifying emergency use of IVDs, unless terminated or revoked (after which the test may no longer be used).
Where can I go for updates and more information?
CDC webpages:
General: https://www.cdc.gov/COVID19
Symptoms:
https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/
symptoms.html
Healthcare Professionals:
https://www.cdc.gov/coronavirus/2019-nCoV/guidance-hcp.
html
Information for Laboratories:
https://www.cdc.gov/coronavirus/2019-nCoV/guidancelaboratories.html
Laboratory Biosafety:
https://www.cdc.gov/coronavirus/2019-nCoV/lab-biosafetyguidelines.html
Isolation Precautions in Healthcare Settings:
https://www.cdc.gov/coronavirus/2019-ncov/infection-control/
control-recommendations.html
Specimen Collection:
https://www.cdc.gov/coronavirus/2019-nCoV/guidelinesclinical-specimens.html
Infection Control:
https://www.cdc.gov/coronavirus/2019-ncov/infection-control/
index.html
Discontinuation of Isolation:
https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-inhome-patients.html
FDA webpages:
General: www.fda.gov/novelcoronavirus
EUAs:(includes links to patient fact sheet and manufacturer’s
instructions) https://www.fda.gov/medical-devices/coronavirusdisease-2019-covid-19-emergency-use-authorizations-medicaldevices/vitro-diagnostics-euas
Abbott Diagnostics Scarborough, Inc.:
10 Southgate Road
Scarborough, Maine 04074
Technical Support:
Telephone: (800) 257 9525
[email protected]
BinaxNOW COVID-19 Ag Healthcare Provider Fact Sheet – Optimized PDF
BinaxNOW COVID-19 Ag Healthcare Provider Fact Sheet – Original PDF
References
]]>
NAVICA for COVID-19
RAPID ANSWERS IN YOUR HANDS TO HELP BRING

The NAVICA™ app is your pass to navigating daily life in a new normal. NAVICA displays results from the 15-minute Abbott BinaxNOW™ COVID-19 Ag Card, an antigen rapid test, to help you and others make informed decisions. NAVICA-enabled COVID-19 test sites are coming soon. Visit NAVICA.ABBOTT to sign up to be one of the first to know when and where the COVID-19 tests are being offered in your community.
How to get a NAVICA Pass
- Download the NAVICA app and create your secure profile.

2.Show your profile’s unique
NAVICA ID to get tested at
a NAVICA-enabled test site

3.Receive a NAVICA Pass when 3 you test negative for COVID-19
WHAT IS NAVICA?
NAVICA is an app that allows you to receive and store your encrypted BinaxNOW™ COVID-19 Ag Card test results and manage your NAVICA Pass.
WHAT IS THE NAVICAPASS?
The NAVICA app will display a digital NAVICA Pass via a QR code, similar to an airline boarding pass, for anyone who has received a negative result from a NAVICAenabled test site using the BinaxNOW COVID-19 Ag Card test. Sharing your pass with NAVICA-enabled organizations such as employers and schools verifies authenticity of your negative COVID-19 test results.
HOW CAN I GET NAVICA?
NAVICA, the first-of-its-kind app, can be downloaded from both the App Store® and Google Play™ at no charge. Go to your app store, search NAVICA and download the blue NAVICA app.
HOW DO I FIND A NAVIC A-ENABLED TEST SITE?
NAVICA-enabled test sites administering the 15-minute
BinaxNOW COVID-19 Ag Card test are coming soon.
Visit NAVICA.ABBOTT to sign up to be one of the first
to know when and where the COVID-19 tests are being
offered in your community.
WHAT KIND OF TEST IS USED?
The BinaxNOW COVID-19 Ag Card is a rapid lateral flow antigen test administered by a healthcare professional or a trained operator. It tests for active infection using a direct nasal swab. It provides a positive or negative result in 15 minutes with no instrument required.
NAVICA BinaxNOW COVID-19 Ag App Instruction Guide – Optimized PDF
NAVICA BinaxNOW COVID-19 Ag App Instruction Guide – Original PDF
References
]]>NAVICA™ MOBILE APP AND BinaxNOW™ COVID-19 AG CARD
TAKING COVID-19 TESTING TO A NEW LEVEL.
Designed to Restore Confidence in Daily Life
The revolutionary NAVICA™ app helps people navigate daily life in a new normal. NAVICA displays results from the 15-minute Abbott BinaxNOW™ COVID-19 Ag Card, an antigen rapid test, to help individuals make informed decisions. This first-of-its-kind app, available at no charge, allows people who test negative to get an encrypted temporary digital NAVICA Pass, similar to an airline boarding pass. NAVICA-enabled organizations will be able to verify an individual’s negative COVID-19 test result by scanning the individual’s digital NAVICA Pass to facilitate entry into facilities, along with hand-washing, social distancing, enhanced cleaning and mask-wearing.
Your Role in the NAVICA Process
As a NAVICA-enabled test site, you will use the NAVICA Administrator app to communicate encrypted BinaxNOW COVID-19 Ag Card test results to participants and allow them to obtain a digital NAVICA Pass with a negative test result. To conduct testing at your site, a CLIA-waived license is required per the FDA Emergency Use Authorization for BinaxNOW COVID-19 Ag Card.
As a BinaxNOW™ COVID-19 Ag Card testing site, you play an
important role in helping people move about with greater confidence.
THE NAVICA™-BinaxNOW TE S TING PRO CE SS I S SIMPLE:
- Scan the participant’s NAVICA ID on their mobile device and verify photo ID match.
- Scan the unique QR code on the BinaxNOW COVID-19 Ag Card to create a secure link between the participant and the test card.
- Perform the BinaxNOW COVID-19 Ag Card test, following the package insert instructions.
- Rescan the QR code on the test card to confirm the link and chain of custody between the BinaxNOW COVID-19 Ag Card test and the participant.
- Visually interpret the test result on the card and submit the positive or negative result accordingly via the NAVICA app.
The participant will be notified of their result submission through the NAVICA app. A negative result submission will generate an encrypted digital NAVICA Pass as a QR code on the individual’s mobile device. If the participant tests positive, they will receive a NAVICA message to follow CDC Guidelines on quarantine and consult their healthcare provider.
Abbott believes the NAVICA app and BinaxNOW COVID-19 Ag Card test will help us recover a bit more freedom, confidence and normalcy in our everyday lives.
BinaxNOW™ COVID-19 AG CARD
A Breakthrough Antigen Test
SIMPLIFYING THE TEST PROCESS
- Cost-effective, high performing test designed for decentralized testing
- Simple test procedure.
—Direct nasal swab.
—Onboard extraction allows the swab to be directly inserted into the test card.
—Visually read results in 15 minutes (no instrument required). - Emergency Use Authorization (EUA) supports testing in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance or Certificate of Accreditation*.
PERFORMANCE
Sensitivity (PPA) 97.1%
Specificity (NPA) 98.5%
TEST PROCEDURE**
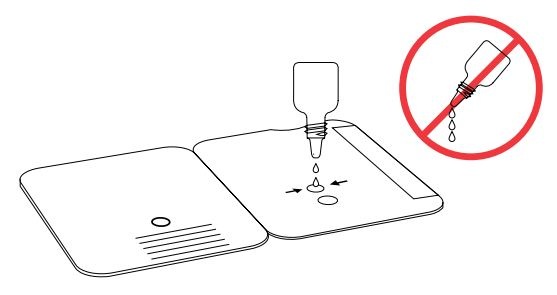
1.Add the extraction
reagent
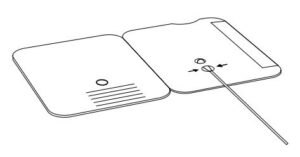
2.Insert the sample
nasal swab
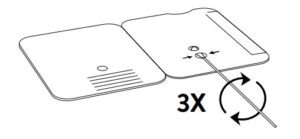
3.Rotate the nasal swab
shaft three times.
4.Close the test card;
wait 15 minutes
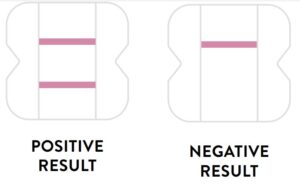
5.Results are read visually
KIT CONTENTS
- 40 Test Cards
- 40 Nasal Swabs
- Positive Control Swab
- Reagent Bottle
- Package Insert
- Procedure Card
- 40 Patient COVID-19 Fact Sheets
- Healthcare Provider COVID-19 Fact Sheet
ORDER INFORMATION

NAVICA APP AVAILABLE FOR DOWNLOAD ON THE APP STORE® AND GOOGLE PL AY™ NAVICA.ABBOTT
NAVICA BinaxNOW COVID-19 Ag Card Product Brochure for Professionals – Optimized PDF
NAVICA BinaxNOW COVID-19 Ag Card Product Brochure for Professionals – Original PDF
References
]]>PROCEDURE CARD
For Use Under an Emergency Use Authorization (EUA) Only.
The BinaxNOW COVID-19 Ag Card is a lateral flow immunoassay for the qualitative detection of the nucleocapsid protein antigen to SARS-CoV-2 directly from nasal swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within seven days of the onset of symptoms.
IMPORTANT: See Product Insert, including QC section, for complete use instructions, warnings, precautions and limitations. False negative results may occur if specimens are tested past 1 hour of collection. Specimens should be tested as quickly as possible after specimen collection. Open the test card just prior to use, lay it flat, and perform assay as follows.
Part 1 – Sample Test Procedure
Patient Samples require 6 drops of Extraction Reagent.
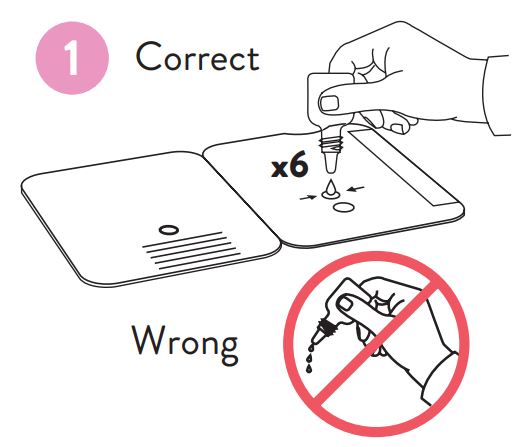
1.Hold Extraction Reagent
bottle vertically. Hovering 1/2
inch above the TOP HOLE,
slowly add 6 DROPS to the
TOP HOLE of the swab well.
DO NOT touch the card
with the dropper tip while
dispensing.
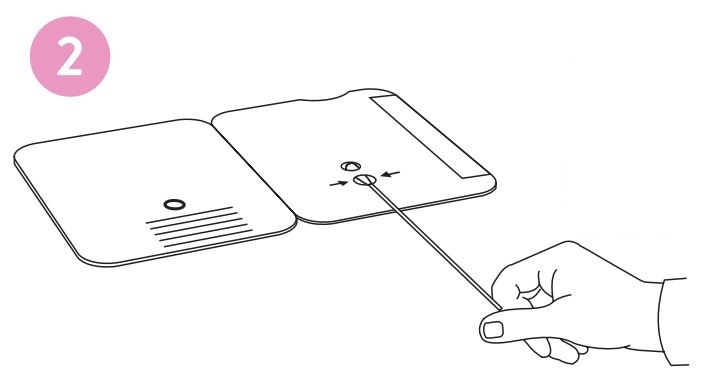
2.Insert sample or control swab
into BOTTOM HOLE and
firmly push upwards so that
the swab tip is visible in the
TOP HOLE
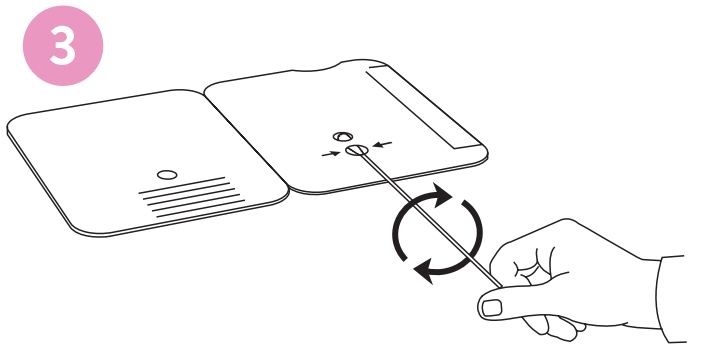
3.Rotate (twirl) swab shaft
3 times CLOCKWISE (to
the right). Do not remove
swab.
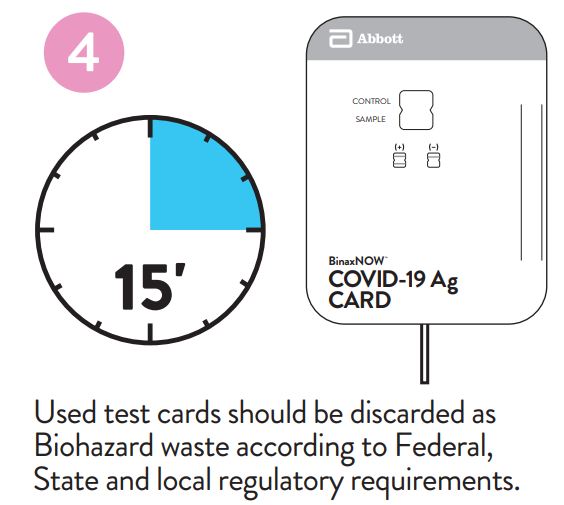
4.Peel off adhesive liner from the right edge of the test card. Close and securely seal the card. Read result in the window 15 minutes after closing the card.In order to ensure proper test performance, it is important to read the result promptly at 15 minutes, and not before. Results should not be read after 30 minutes.
Part 2 – Result Interpretation
Negative Result
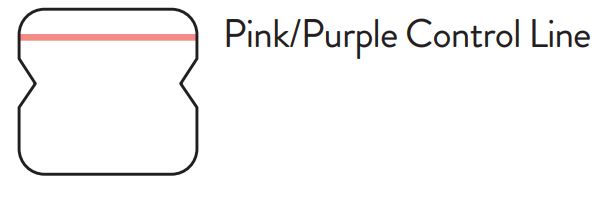
A negative specimen will give a single pink/purple colored Control Line in the top half of the window, indicating a negative result. This Control Line means that the detection part of the test was done correctly, but no COVID-19 antigen was detected. Negative results, from patients with symptom onset beyond seven days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed.
Positive Result
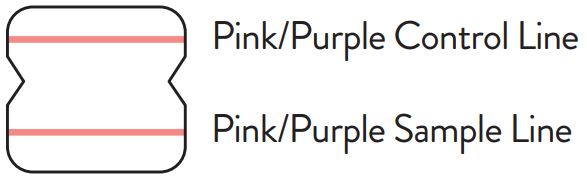
A positive specimen will give two pink/purple colored lines. This means that COVID-19 antigen was detected. Specimens with low levels of antigen may give a faint Sample Line. Any visible pink/purple colored line is positive.
Invalid Result
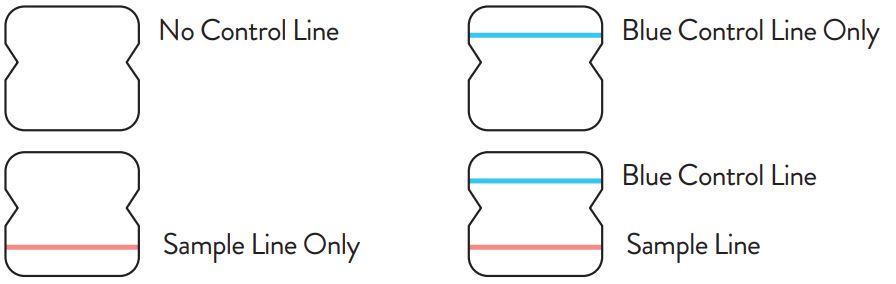
If no lines are seen, or if just the Sample Line is seen, the assay is invalid. Invalid tests should be repeated.
Procedure for External Quality Control Testing
External Controls require 8 drops of Extraction Reagent
- Hold Extraction Reagent bottle vertically. Hovering 1/2 inch above the TOP HOLE, slowly add 8 DROPS to the TOP HOLE of the swab well. DO NOT touch the card with the dropper tip while dispensing.
- Follow Steps 2 – 4 of the Test Procedure shown.
In the USA, this test has not been FDA cleared or approved; this test has been authorized by FDA under an EUA for use by authorized laboratories; use by laboratories certified under the CLIA, 42 U.S.C. §263a, that meet requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or
Certificate of Accreditation. This test has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. In the USA, – this test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of the virus that causes COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.

Technical Support Advice Line
Further information can be obtained from your distributor, or by contacting Technical Support on: US +1 800 257 9525 [email protected]
Abbott Diagnostics Scarborough, Inc.
10 Southgate Road Scarborough, Maine 04074 USA
IN195001
BinaxNOW COVID-19 Ag Procedure Instruction Card – Optimized PDF
BinaxNOW COVID-19 Ag Procedure Instruction Card – Original PDF
References
]]>Perform the BinaxNOW™ COVID-19 Ag Card test and communicate encrypted test results using the NAVICA™ Administrator App.
1.Log in to the NAVICA™ Administrator
App, and select Begin New Test.
2.Scan the participant’s unique NAVICA ID
QR code using the camera on your tablet.
3.Verify the participant’s
identity with a photo ID.
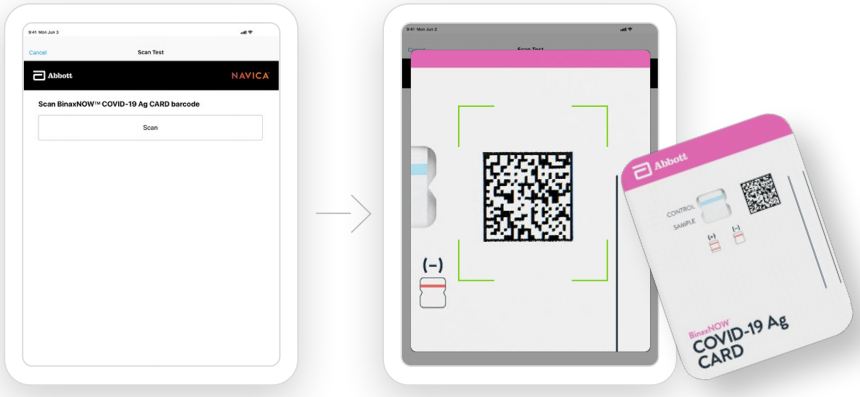
4.Scan the BinaxNOW™ COVID-19 Ag Card QR code to link the participant’s NAVICA ID to the test card.

5.Collect the nasal swab
and perform the test. (See the BinaxNOW™ COVID-19 Ag Card Product Insert, including the quality control section, for complete use instructions, warnings, precautions and limitations.)
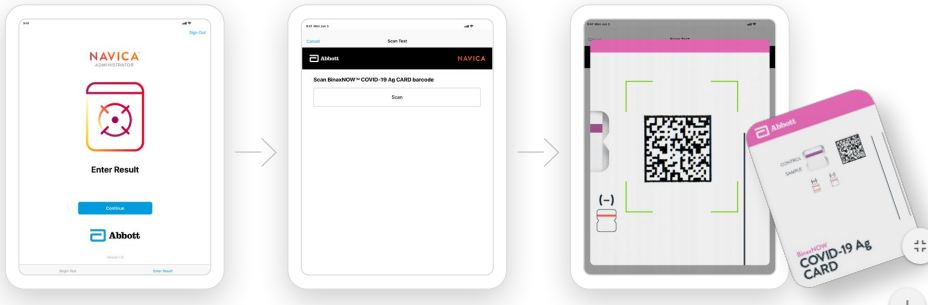
6.Collect the nasal swab
and perform the test. (See the BinaxNOW™ COVID-19 Ag Card Product Insert, including the quality control section, for complete use instructions, warnings, precautions and limitations.)
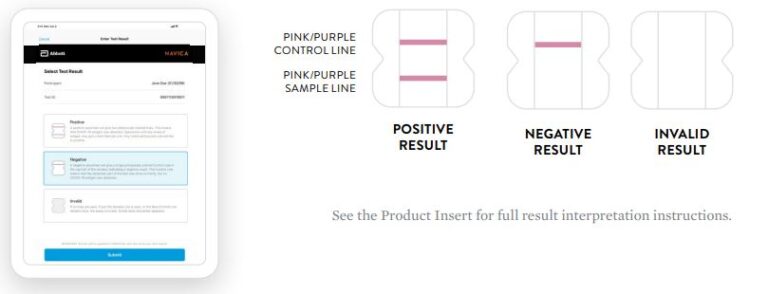
7.Visually interpret the results on the test card and enter the results in the NAVICA Administrator App with a
single tap.
THE PARTICIPANT WILL RECEIVE A PUSH NOTIFICATION AND BE NOTIFIED VIA EMAIL TO REVIEW THEIR ENCRYPTED RESULT IN THE NAVICA APP. YOUR FACILITY WILL SEPARATELY NOTIFY THE PARTICIPANT VIA YOUR REGULAR PROCESS FOR LAB TEST REPORTING.
BinaxNow NAVICA App Administrator Quick Reference Guide – Optimized PDF
BinaxNow NAVICA App Administrator Quick Reference Guide – Original PDF
References
]]>
BinaxNOW COVID-19 At Home Test Instruction Manual

- This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARSCoV-2, not for any other viruses or pathogens; and,
- This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/r diagnosis of COVID-19 under Section 564(b (1)federalization,Drug and Cosmetic Act,21 U.S.C.§360bbb-3(b)(1),unless the declaration is terminated or authorization is revoked sooner.
Will this testhurt?
No, the nasal swab is not sharp and it should not hurt. Sometimes the swab can feel slightly uncomfortable or tickly. If you feel pain, please stop the test and seek advice from a healthcare provider.
What are the known and potential risks and benefits of this test?
Potential risks include:
- Possible discomfort during sample collection.
- Possible incorrect test results (see Results section).
Potential benefits include:
- The results, along with other information, can help your healthcare provider make informed recommendations about your care.
- There results of this test may help limit the spread of COVID-19 to your family and others in your community.
You have the option to refuse this test. However, your doctor has prescribed this test because they believe it could help with your care.
What is the difference between an antigen and molecular test?
There are different kinds of tests for COVID-19. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests detect proteins from the virus. Antigen tests are very specific for the virus, but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. If your test result is negative, you should discuss with your healthcare provider whether an additional molecular test would help with your care, and when you should discontinue home isolation.
How Accurate is this Test?
Based on the interim results of a clinical study where the BinaxNOWTM COVID-19 Ag Card Home
Test was compared toan FDA authorized high sensitivity SARS-CoV-2test,BinaxNOW COVID-19 Ag Card Home Test correctly identified 91.7% of positive specimens and 100% of negative specimens.
BinaxNOW COVID-19 Ag Card Home Test Study*

In the graph above, BLUE bars represent samples where the both the BinaxNOW COVID-19 Ag Card Home Test and molecular test result were positive. The ORANGE bars represent samples where the BinaxNOW COVID-19 Ag Card Home Test results that were negative and the molecular test was positive. All of the ORANGE bars occurred in samples where there were very low levels of virus detected by the molecular test. In a separate larger study, the BinaxNOW COVID-19 Ag Card clinical study where tests were performed by healthcare providers, BinaxNOW COVID-19 Ag Card correctly identified 90.6% (116/128) positive specimens with high amounts of virus.
Due to the relatively small sample size for the home use clinical study, the BinaxNOW™ COVID-19 Ag Card Home Test is estimated to correctly identify between 73.0% and 98.9% of positive specimens as reflected in the 95% Confidence Interval. This is consistent with the performance established in a separate multi-site clinical study in the US, where the BinaxNOW COVID-19 Ag Card test was performed and results interpreted by test operators with no laboratory experience. In that study, BinaxNOW™ COVID-19 Ag Card test correctly identified 84.6% of positive specimens
and 98.5% of negative specimens.
Based on this information, negative results may require additional testing to confirm your result.
Please talk to your healthcare provider to determine if you need additional testing.
Indication
The Binax NOW COVID-19Ag Card Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This tests authorized for prescription home use with self-collected observed direct anterior nasal (nares) swab samples from individuals aged 15 years or older who are suspected of COVID19 by their healthcare provider within the first seven days of symptom onset or adult-collected nasal swab samples from individuals aged four years or older whoa re suspected of COVID-19by their health care provider with in the first seven days of symptom onset. The BinaxNOWCOVID-19AgCard Home Test is to be performed only with the supervision of a tele health proctor.
TheBinaxNOWCOVID-19AgCard Home Test does not differentiate between SARS-CoV and SARS-CoV 2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal (nares) swabs during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation
with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial in fectionorco-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results should be treated as presumptive and confirmation with a molecular assay,if necessary,for patient management, may be performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures,history and the presence of clinical signs and symptoms consistent with COVID-19. individuals who test negative and continue to experience COVID-like symptoms should seek follow up care from their healthcare provider. BinaxNOWCOVID-19AgCard Home Test isonly foruse under the Food and Drug Administration’s Emergency Use Authorization. All prescribing healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC
and SNOMED codes,as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC.
Wash Your Hands
Wash or sanitize your hands. Make sure they are dry before starting.

1.Set Up
DO NOT open items until instructed.
A.Open your test kit
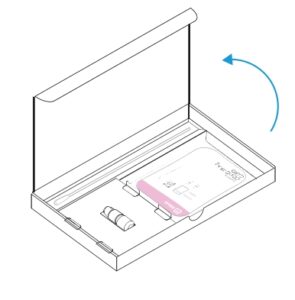
B.You should have:
2.Open Pouch and Scan QR Code on Card
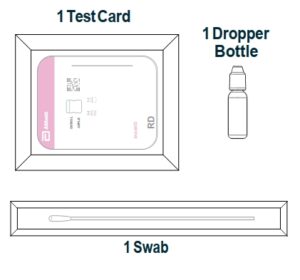
3.Open Card

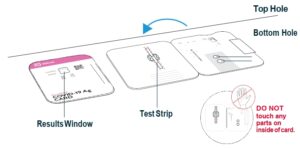
4.Apply Fluid to Top Hole
A. Remove dropper bottle cap.
B. Hold dropper bottle straight over TOP HOLE, not at an angle.
C. Put 6 DROPS into TOP HOLE. Do not touch card with tip
5.Open Swab
A. Open swab package at stick end.
B. Take swab out.

6.Swab Left Nostril
A. Insert the entire absorbent tip of the swab (usually 1/2 to 3/4 of an inch) into left nostril.
B. Firmly brush against insides of nostril in a circular motion 5 times or more for at least 15 seconds.


7.Swab Right Nostril
A. Remove swab and insert it into right nostril.
B. Firmly brush against insides of nostril in a circular motion 5 times or more for at least 15 seconds.

8.Insert Swab Into Bottom Hole
Insert swab tip into BOTTOM HOLE and firmly push up until tip fills TOP HOLE

9.Turn Swab 3 Times
Turn swab to right 3 times in card and leave it in place

10.Peel Strip
Keep swab in place. Peel adhesive liner off.

11.Close Card And Seal
Close left side of card over swab to sea lit. Keep card face up on table.

12.Wait 15 Minutes


Note: False results can occur if the card is disturbed/moved or test results are read before 15 minutes.
13.Scan QR Code

14.Show Result to YourProctor

15. Steps To Check Your Results
There are three types of results possible. You will be instructed how to read each type in a specific order. Follow this order with your proctor:
- Check for a Positive Result
- Check for a Negative Result
- Check for an Invalid Result
16.Check for Positive COVID-19 Result
Find result window and look carefully for two pink/purple lines in window.
•Positive Result: Two pink/purple lines will appear. One on the top
half and one on the bottom half. COVID-19 was detected.

Here are photos of actual positive tests. On the right, note how faint the bottom line can get.

Positive COVID-19Result
A positive test result mean sit is very likely you have COVID-19 and it is important to be under the care of your health care provider.
The telehealth proctor is not a healthcare provider. It is also
likely that you may be placed in isolation to avoid spreading the
virus toothers. There is a very small chance that this test can give
a positive result that is wrong (a false positive result). Your
healthcare provider will work with you to determine how best to
care for you based on your test result(s) along with your medical
history, and your symptoms.
17.Check for Negative COVID-19 Result
Find result window and look for a single pink/purple line in window.
• Negative Result: A single pink/purple line on the top half
where it says“Control.”COVID-19 was not detected.

Negative COVID-19Result
A negative test result means that proteins from the virus that
causes COVID-19 were not found in your sample.
Negative results may require additional molecular
testing to confirm that you do not have COVID-19.
It is possible for this test to give a negative result that is incorrect
(false negative)in some people with COVID-19. This means that
you could possibly still have COVID-19 even though the test is
negative. If your test result is negative, please consult your
healthcare provider. The telehealth proctor is not a
healthcare provider. Your health care provider will consider the
test result together with all other aspects of your medical
history (such as symptoms, possible exposures, and
geographical location of places you have recently traveled)in
deciding how to care for you. If you or the healthcare proctor disagree on the presence of a faint line and/or the presence of a line is uncertain, additional BinaxNOWCOVID 19Ag CARD HOME TEST KIT confirmatory testing should be conducted. It is important that you work with your health care provider to help you understand the next steps you should take.
18.Check for Invalid Result
If you see any of these, the test is invalid.

19.Dispose in Trash

Reporting Patient Results Using NAVICA
Up on completion of the test and result interpretation, the telehealth proctor will send your test results via the NAVICA app. You will be notified by email and on your mobile device that your results are ready. You will go to the results screen in NAVICA to obtain your test results.

Reporting Patient Results Using NAVICA
Upon completion of the test and result interpretation, the telehealth proctor will send your test results via the NAVICA app. You will be notified by email and on your mobile device that your results are ready. You will go to the results screen in NAVICA to obtain your test results.
If the BinaxNOW COVID-19 Ag Card Home Test result is In valid,you will receive the following:

BinaxNOW COVID-19 At Home Test Instruction Manual – Optimized PDF
BinaxNOW COVID-19 At Home Test Instruction Manual – Original PDF
FAQS
Abbott, not customers: what is the difference between this antigen self test and the binaxnow covid-19 ag card home test?
The BinaxNOW COVID-19 Antigen Self Test does not currently pair with the NAVICA app. The BinaxNOW COVID-19 Home Test can provide a sharable digital COVID-19 result, through collaboration with eMed. The BinaxNOW COVID-19 Antigen Self Test is the identical test card to the professional test but can be purchased at retail locations without a prescription. There’s also no need for a healthcare professional or online proctor to administer the swab or interpret results. Please let us know if there is anything else we can help you with.
Waiting for customer reviews. How accurate is the test?
This is an antigen test, not as accurate as a PCR molecular test. Depends on the viral load in your nasal passages. If positive you are likely infected with Covid. If negative and have symptoms test again and get a PCR test.
does it test for antibodies?
No, this is an antigen test that only detects an active infection. Antibody tests can detect past infections. Please let us know if there is anything else we can help you with.
What are the specificity and sensitivity ratings for this test?
Based on the interim results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
False positive and false negative rates for the test?
Based on the interim results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
To mfg: So many customers getting unsealed boxes–are new tests being put in old boxes? My lot # on box doesn’t match the lot # on tests inside
my box was sealed and a new test. The reactant agent, not sure if that is what you call it was sealed as well. I ordered two boxes, so 4 test total, did not have an issue.
Can this test be administered through the mouth with saliva rather than the nose?
Our BinaxNOW Self Test is simple. Each test kit includes step-by-step instructions and a quick reference guide with illustrations. You perform a minimally invasive nasal swab and all materials required to perform the test come in the box, with the exception of a timer.
Is this item hsa/fsa eligible?
That’s up to individual insurance companies to determine. Some cover other Over-the-Counter products so individuals would need to reach out to their provider to determine coverage.
Why did Abbott stop making this test?
We are producing millions of rapid tests per month here in the U.S. and, as we have been throughout the entire pandemic, are committed to making rapid tests available to more people at affordable prices and convenient places.
What is the QR code on the results card for?
The QR code on a BinaxNOW COVID-19 test card is used by NAVICA, Abbott’s COVID-19 digital testing system. NAVICA can be used to easily capture, manage and share self reported results from the BinaxNOW COVID-19 Antigen Self Test. It is available at no cost on the App Store or Google Play.
How are the swabs sterilized?
They come in a sealed packet
is there any part of the test kit that would get confiscated at TSA airport screening
The BinaxNOW COVID-19 Antigen Self Test can be taken on airplanes as there is nothing hazardous in the kit and the ampoule of extraction reagent is well under the 3 ounce liquid restriction.
Is it able to detect breakthrough infections in vaccinated people?
We are monitoring the mutations of the COVID-19 virus to ensure our tests can detect them, as we do with many viruses. We have conducted a thorough analysis of known variants that we’ve been able to study, and we are confident that our tests remain effective at identifying these strains.
For Abbot only, how accurate is this test (expressed as a sensitivity percentage) at detecting Covid-19 in an asymptomatic individual?
Based on the results of a clinical study where the BinaxNOW™ COVID-19 Antigen Self Test was compared to an FDA authorized high sensitivity SARS-CoV-2 test, BinaxNOW COVID-19 Antigen Self Test correctly identified 84.6% of positive specimens and 98.5% of negative specimens.
What do I do if my nasal passages are blocked?
If your nasal passages are blocked, please follow these steps:
1) Blow your nose before you take the test.
2) Take a decongestant (such as SudafedTM) at least 30 minutes before you take the test.
3) Take a decongestant (such as SudafedTM) after you have taken the test.
4) If you still have trouble breathing, contact your healthcare provider.
What do I do if I am sick?
If you are sick, please follow these steps:
1) Wait until you are better before taking this test.
2) Take a decongestant (such as SudafedTM) at least 30 minutes before you take this test.
3) Take a decongestant (such as SudafedTM) after you have taken this test.
4) If you still have trouble breathing, contact your healthcare provider.
![]()






