AMBRY COVID-19 RT-PCR TEST
Saliva Self-Collection & Shipping Instructions
Please follow the directions below to collect your COVID-19 testing sample, which was recommended by a physician for you to complete.
- IMPORTANT: Read all instructions thoroughly before you start to ensure proper sample collection for testing.
- Do NOT eat, drink or chew gum for 30 minutes prior to collecting your sample.
- Do NOT remove the plastic film from the funnel lid until you have collected the sample.
- You MUST register your kit and collect your sample on the SAME day that you ship it. Do not collect on Saturday or Sunday. You can also watch a video on sample collection steps at: ambrygen.com/salivacollection
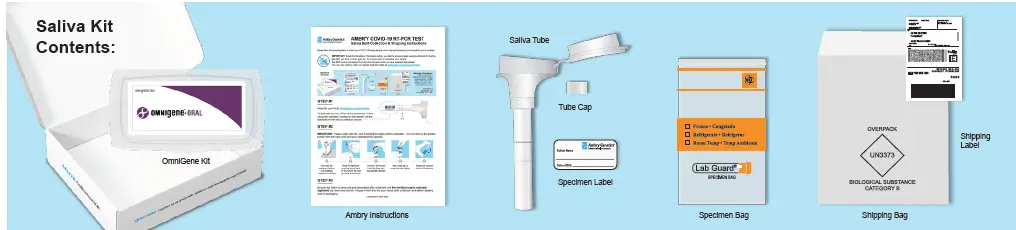
Saliva Kit Contents:
Warnings & Precautions:
Keep out of reach of children when not in use. Use of gloves and protective eyewear is recommended (not provided in the kit). Do not ingest the stabilizing liquid. Adults should pay attention while assisting/supervising collection from children to avoid accidental exposure to the stabilizing liquid. Wash with water if the stabilizing liquid comes into contact with the skin or eyes. Seek medical advice if irritation persists. Small-cap may pose a choking hazard. See MSDS at dnagenotek.com. Kit Storage: 15̊ C – 30̊C.
STEP #1
Register your kit at ambrygen.com/covidkit
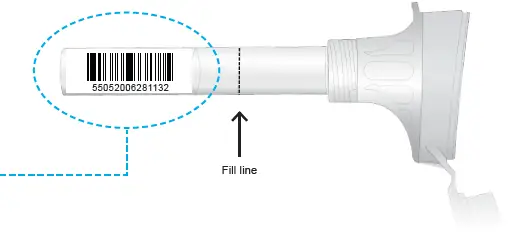
To activate your kit, follow the instructions online using the numbers located on the bottom of the barcode on the saliva collection device.
STEP #2
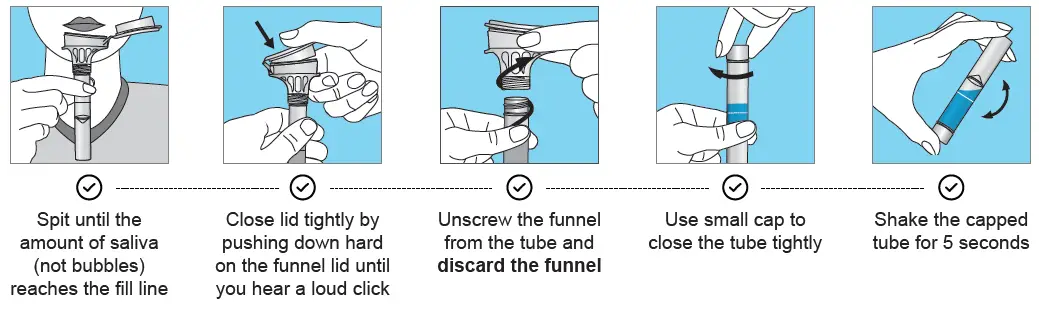
IMPORTANT: Please wash and dry your hands thoroughly before collection. Do not remove the plastic funnel from the tube until you have collected the sample. Adults assisting collection from children should perform all downstream steps after saliva collection.
STEP #3
Ensure the funnel is removed and discarded after collection and the enclosed cap is securely tightened (as described above). Please wash and dry your hands after collection and before labeling and re-packaging.
STEP #4
Write your first and last name, and date of birth (MM/DD/YYYY) on the small Ambry sticker where indicated
Affix the naming label on back of the tube as illustrated on the white rectangle on back of tube. Ensure the naming sticker does not cover the barcode on the tube.
NOTE: Testing may be delayed or not performed if the tube is received unlabeled, leaking, or damaged.
STEP #5
- Place the closed saliva tube in the biohazard bag; Do not remove the absorbent material from the biohazard bag.
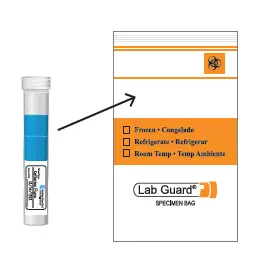
- Seal the bag and place it inside the cardboard box where the saliva collection kit was originally located.

- Place the cardboard box in the shipping bag that is already affixed with a FedEx return label; then seal the package.
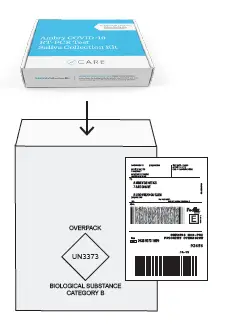
STEP #6
Please drop off the package before the last pick-up of the day, Monday – Friday at a FedEx dropbox. For locations, search by zip code at: fedex.com/en-us/shipping/dropbox.html
- FOR EMERGENCY USE AUTHORIZATION ONLY
- FOR PRESCRIPTION USE ONLY
- FOR IN VITRO DIAGNOSTIC USE
- For Use by individuals 18 years of age and older when self-collected
- For Use by individuals 15 years of age and older when self-collected under adult supervision
- For Use by individuals 4 years of age and older when collected with adult assistance
This product has not been FDA cleared or approved but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories.
This product has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for the detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetics Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For questions on how to send your sample to Ambry Genetics,
please contact our Customer Service team at [email protected]