Lateral Flow Method
Nasal Swab Test for selftesting use – Temporary special approval after §11 MPG in Germany (BfArM GZ: 5640-S-179/21)
Please read the instructions for use carefully before performing the selftest. Please watch before using the selftest the explanatory film on http://en.wondfo.com.cn/resource/index.html.
INTENDED USE
The 2019-nCoV Antigen Test (Lateral Flow Method) is an immunochromatographic assay for rapid, qualitative detection of novel coronaviruses (2019-nCoV) antigen extracted from the nasal swab specimen. The test is to be used as an aid in the diagnosis of coronavirus infection disease (COVID-19), which is caused by 2019-nCoV. The test provides preliminary test results. Negative results cannot exclude 2019-nCoV infection and they cannot be used as the sole basis for treatment or other management decision.For in vitro diagnostic use only. Under the temporary special approval after §11 MPG in Germany (BfArM GZ: 5640-S-179/21) it may be used by laypersons for selftesting. Suitable for ages from 18 years. However, for the users age under 18, it is recommended to perform the test under adult supervision, while the users age over 70 should be aware of the removal of their nasal swab or have nasal swabs assist.
SUMMARY
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious
disease. People are generally susceptible. Currently, the patients infected by the novel corona- virus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The 2019-nCoV Antigen Test (Lateral Flow Method) is based on the principle of Imm nochromatography sandwich for determination of 2019-nCoV Nucleocapsid antigen extract-ed from the nasal swab specimen. When the extracted specimen is added into the test device, the specimen is absorbed into the device by capillary action, reacts with the 2019-nCoV antibody-dye conjugate and flows across the pre-coated membrane. The test Region (T) of this device is coated with antibody against Nucleocapsid protein of 2019-nCoV, while the AuNPs are modified by another antibody against Nucleocapsid protein of 2019-nCoV, and the conjugate spread on the pad. When the 2019-nCoV antigen level in the specimen is at or above the target cutoff (the detection limit of the test), the antigen bound to the antibody- AuNPs conjugate are combined by 2019-nCoV antibody immobilized in the Test Region (T) of the device, and this produces a colored test band that indicates a positive result. When the 2019-nCoV antigen level in the specimen is zero or below the target cutoff, there is not a visible colored band in the Test Region (T) of the device. This indicates a negative result.
The control Region (C) of this device is coated with goat-anti-mouse antibody, to serve as a
procedure control, a colored line will appear at the Control Region (C), if the test has been
performed properly.
PRECAUTION
- The kit is intended for in vitro diagnostic use only.
- All smears should be treated as potential carriers of the disease. Take all necessary precautions for the collection, handling, storage and disposal of swabs and used kit contents.
- Unless you are using the test on yourself, wear an FFP2 or medical face mask and safety glasses when handling.
- Proper extraction of nasal swab specimens and accurate performance of the test procedure are critical to the result of the test.
- Dispose of all used products after single use with the help of the disposal bag.
- Avoid excessive temperatures in the test environment. Test cassettes and extraction solution stored at low temperatures must be returned to room temperature before opening in order to avoid condensation of moisture. Do not touch the reaction area of the test strip (openings of the test cassette).
- Do not use the test kit after the expiration date.
- If the pouch is damaged or the seal is broken, do not use the kit.
- If you have signs of COVID-19 despite a negative result, you should
Test result to be checked by a doctor. - Hold the nasal swab by the style, not the padded end.
- This kit is for external use. Don’t swallow.
- Avoid getting the buffer solution in contact with eyes or skin.
- Keep out of the reach of children.
- Do not use this test beyond the expiration date stated on the outer packaging
out. Always check the expiration date before testing. - Avoid getting the extraction solution in your eyes or on your skin.
- DISPOSAL OF THE DIAGNOSTICS: All components used pose a potential risk of infection. Dispose of them using the disposal bag provided. Stow all the products and materials you have used in it, then close the bag and throw the bag in the trash.
STORAGE AND STABILITY
- The products can be stored at + 2 ° C to + 30 ° C up to the one printed on the packaging Expiry date to be transported, stored and used.
- Products must not freeze or freeze!
- The test cassette must be transported and stored in the sealed pouch.
- The test device should be used within one hour of removing it from the sealed pouch.
- Protect from sunlight, moisture and heat.
- The date of manufacture is printed on the packaging.
MATERIALS

Materials Required but Not Provided
- Timer (Watch, Clock)
- Personal protective equipment (e.g. PPF2 or medical mask, goggles) when handing the contents of this kit for another person.
SPECIMEN COLLECTION AND PREPARATION
Bring the test components to room temperature (15 to 30 ° C).
For this test, choose a location where you can do the test for 20 minutes as undisturbed as possible can perform.
Wash or disinfect your hands before performing the testing process.
The test can be performed with a nasal swab sample.
If possible, blow your nose before use. This can reduce the reliability of the Increase tests.
- Hold the nasal swab by the notch.
- Tilt the head (of the test person) backwards (approx. 70 degrees).
- While gently rotating the swab, guide the entire padded tip of the nasal swab about 1.5 to 2 cm into one nostril.
- Take the first swab by using the nasal swab relatively forcefully to swab the Rub the inside of the nostrils, making a circular five times against the walls of the nose Turn so that the padded surface of the nasal swab is moistened all around. Notes: a) But it shouldn’t hurt. b) Take about 15 seconds to do this Take action.
- Slowly remove the nasal swab from the first nostril.
- Repeat the process with the same nasal swab in the other nostril.
TEST PROCEDURE
TEST PROCEDURE – I ( with Extraction Buffer Tube) For W634P0020/W634P0021/W634P0022/W634P0023
1. Nasal swab specimen extraction
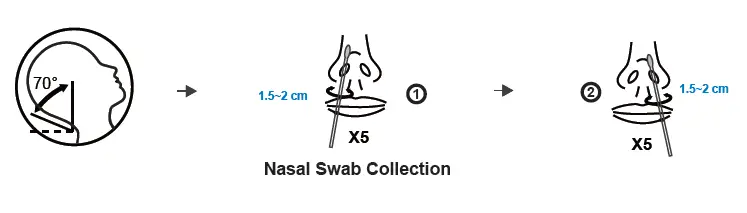
(a) Transfer all of the extraction solution into the sample tube.
(b) Insert the padded nasal swab tip, moistened with nasal secretion, into the sample tube and twist the nasal swab tip against the bottom and sides of the sample tube ten times to loosen the secretion from the nasal swab tip. Place the sample tube in the sample tube holder and let the swab stand in the extraction solution for 1 minute.
(c) Take the sample tube again in one hand and squeeze it together. With the sample tube squeezed, slowly pull the nasal swab out to extract as much of the liquid as possible from the tip of the nasal swab.
(d) Place the dropper tip on the sample tube.
2. Test procedure
(e) Tear open the sealed pouch at the incision, remove the test cassette and place it on a flat surface.
(f) Turn the sample tube upside down, hold it vertically and give 3, maximum 4
Drop into the small, round, white sample well without any inscription. Start the timer.
The result window (C T) now turns purple and a line is formed on the C line and, if necessary, a second line on the T line.
(g) Even if a first line usually appears after approx. 3 minutes, please wait 15 minutes after point (f) and only then read off the test result. This result is valid. After a further 5 minutes (more than 20 minutes after point (f), the result may change again in rare cases. The result counts 15-20 minutes after point (f).
TEST PROCEDURE – II with Pre-installed Extraction Buffer) For W634P0024/W634P0025/W634P0026/W634P0027
1. Nasal swab specimen extraction
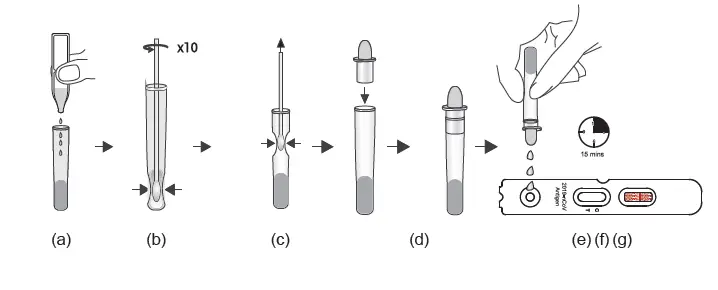
(a) Unscrew the cover of the pre-filled sample tube.
Insert the padded nasal swab tip moistened with nasal secretion into the sample tube and twist the nasal swab tip ten times against the bottom and sides of the sample tube to loosen the secretion from the nasal swab tip.
(b) Place the nasal swab with the padded side in the sample tube. Break off the tip of the nasal swab at the first stop (notch). The padded tip of the nasal swab remains in the sample tube.
(c) Then unscrew the lid. Place the sample tube in the sample tube holder and let the swab stand in the extraction solution for 1 minute.
(d) Remove the cap from the lid.
2. Test procedure
(e) Tear open the sealed pouch at the incision, remove the test cassette and place it on a flat surface.
(f) Turn the sample tube upside down, hold it vertically and give 3, maximum 4 Drop into the small, round, white sample well without any inscription. Start the timer.
The result window (C T) now turns purple and a line is formed on the C line and, if necessary, a second line on the T line.
(g) Even if a first line usually appears after approx. 3 minutes, please wait 15 minutes after point
(f) and only then read off the test result. This result is valid. After a further 5 minutes
(more than 20 minutes after point (f), the result may change again in rare cases. The result counts 15-20 minutes after point (f).
4. The test result of the reagent is used for clinical reference only and should not be used as the only one Can be used as a basis for diagnosis and treatment.
5. The clinical management of patients should be comprehensive based on their Symptoms / signs, medical history, other laboratory tests and their responses
treatment should be considered.
6. Due to the limitations of the method of antigen test reagents used for verification
and confirmation of negative test results the use of determination methods
recommended based on nucleic acid detection or virus culture.
7. Positive test results do not rule out the possibility of co-infection with other pathogens out. A negative result of the reagent can be caused by:
- improper sampling, improper transfer or handling of Samples, virus titer too low in the sample;
- The amount of 2019-nCoV antigen is below the detection limit of the test.
- Variations in the viral genes can lead to changes in the antigen determinants.
PERFORMANCE CHARACTERISTICS
A. Sensitivity and Specificity
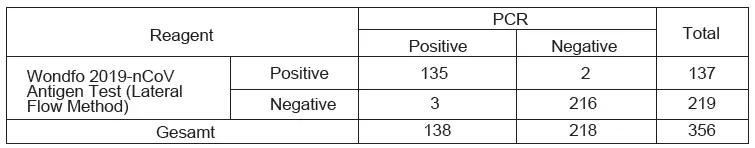
356 clinical case samples (including symptomatic and asymptomatic cases) which include 138 confirmed as COVID-19 positive and 218 confirmed as COVID-19 negative by PCR assay, were obtained for testing, and then compared the test results between Wondfo 2019-nCoV Antigen Test (Lateral Flow Method) and the PCR results. The results are shown below.
- Sensitivity: 97.83% (95%CI: 93.78%~99.55%)
- Specificity: 99.08% (95%CI: 96.73%~99.89%)
- Total agreement: 98.60% (95%CI: 96.75%~99.54%)
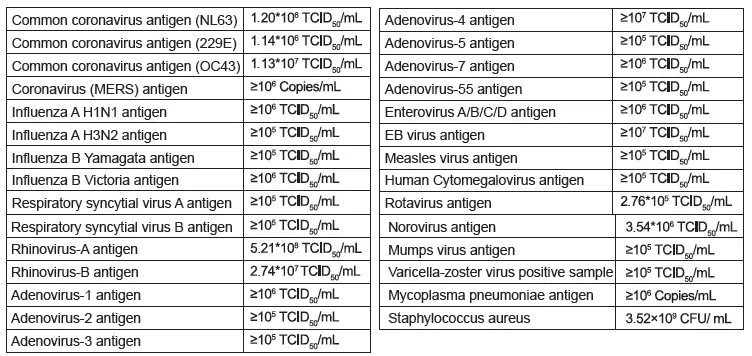
B. Cross-reactivity
Cross-reactivity of the Wondfo 2019-nCoV Antigen Test (Lateral Flow Method) was evaluated using specimens containing the antigens listed below. The results showed no cross-reactivity with the following:
C. Hook effect
Within the titer range of clinically positive samples of 2019-nCoV antigens, there is no hook effect in the test results of this product
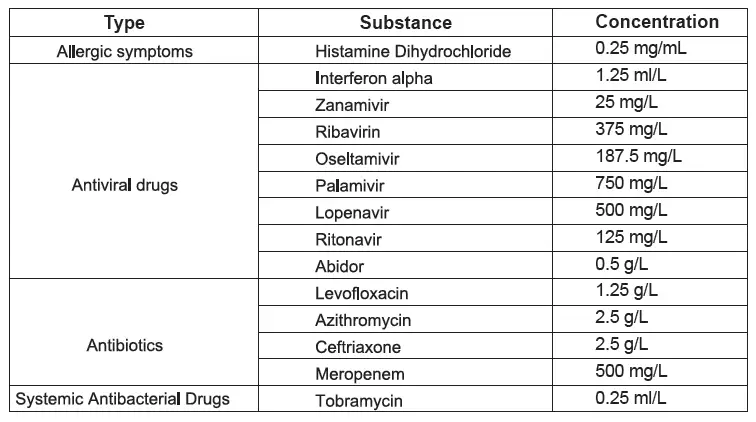
D. Interference
The test result of Wondfo 2019-nCoV Antigen Test (Lateral Flow Method) do not be interfered with the following substance:
It is not known whether other drugs will affect the test result.
If you have any questions, please contact your doctor or pharmacist and read the information leaflet that came with your medication before using the test.
Other possible risks of this test:
- Discomfort during the smear
- If you swallow extraction liquid, drink some liquid. If you get them in your eyes, rinse them out with water.
E. Precision
- Within run precision was determined by testing positive specimens in 10 times. The agreement rate was 100%.
- Between run precision was determined by testing three different specimens including positive and negative in 3 different lots of test devices. The negative agreement rate and the positive agreement rate were 100%.
F. Limit of Detection
The LoD of this test is 1.1×10² TCID50/mL
BIBLIOGRAPHY
Chen H , Wurm T , Britton P , et al. Interaction of the Coronavirus Nucleoprotein with Nucleolar Antigens and the Host Cell[J]. Journal of Virology, 2002, 76(10).
INDEX OF SYMBOL
Guangzhou Wondfo Biotech Co., Ltd. No. 8 Lizhishan Road, Science City, Luogang District, 510663 Guangzhou, P.R.China
Tel: (+86) 400-830-8768
Website: www.wondfo.com.cn
E-mail: [email protected]
Test for selftesting use – Temporary special approval after §11 MPG in Germany (BfArM GZ: 5640-S-179/21)
]]>