CONTEC CMS50DL Pulse Oximeter

Instructions to User
Dear users, thank you very much for purchasing the Pulse Oximeter (hereinafter referred to as device). This Manual is written and compiled in accordance with the council directive MDD93/42/EEC for medical devices and harmonized standards. In case of modifications and software upgrades, the information contained in this document is subject to change without notice. It is a medical device, which can be used repeatedly. The Manual describes, in accordance with the device’s features and requirements, main structure, functions, specifications, correct methods for transportation, installation, usage, operation, repair, maintenance and storage, etc. as well as the safety procedures to protect both the user and device. Refer to the respective chapters for details.
Please read the User Manual carefully before using this device. The User Manual which describes the operating procedures should be followed strictly. Failure to follow the User Manual may cause measuring abnormality, device damage and human injury. The manufacturer is NOT responsible for the safety, reliability and performance issues and any monitoring abnormality, human injury and device damage due to users’ negligence of the operation instructions. The manufacturer’s warranty service does not cover such faults. Owing to the forthcoming renovation, the specific products you received may not be totally in accordance with the description of this User Manual. We would sincerely regret for that. Our company has the final interpretation to this manual. The content of this manual is subject to change without prior notice.
Warnings
Remind that it may cause serious consequences to tester, user or environment.
- Explosive hazard—DO NOT use the device in environment with inflammable gas such as anesthetic.
- DO NOT use the device while examining by MRI or CT, as the induced current may cause burn.
- Do not take the information displayed on the device as the sole basis for clinical diagnosis. The device is only used as an auxiliary means in diagnosis. And it must be used in conjunction with doctor’s advice, clinical manifestations and symptoms.
- The maintenance to the device. Users are not permitted to maintain or refit the device by themselves.
- Uncomfortable or painful feeling may appear if using the device ceaselessly, especially for the microcirculation disturbance users. It is not recommended that the sensor is used on the same finger for more than 2 hours.
- For some special users who need a more careful inspection on the test site, please don’t place the device on the edema or tender tissue.
- Please do not stare at the red and infrared light emitter (the infrared light is invisible) after turning on the device, including the maintenance staff, as it may be harmful to the eyes.
- The device contains silicone, PVC, TPU, TPE and ABS materials, whose biocompatibility has been tested in accordance with the requirements in ISO 10993-1, and it has passed the recommended biocompatibility test. The person who is allergic to silicone, PVC, TPU, TPE or ABS can not use this device.
- Do NOT strand the lanyard to avoid device drop and damage. The lanyard is made of insensitive material. Please do not use it if any person is allergic to lanyard. Do not wrap the lanyard around neck to avoid an accident.
- The disposal of scrap device, its accessories and packaging should follow the local laws and regulations, to avoid polluting to the local environment. And the packaging materials must be placed in the region where the children are out of reaching.
- The device can not be used with the equipment not specified in the Manual. Only the accessories appointed or recommended by the manufacturer can be used, otherwise it may cause injury to the tester and operator or damage to the device.
- Check the device before use to make sure that there is no visible damage that may affect user’s safety and device performance. When there is obvious damage, please replace the damaged parts before use.
- Functional testers can not be used to assess the accuracy of the Pulse Oximeter.
- Some functional testers or patient simulators can be used to verify whether the device works normally, for example, INDEX-2LFE Simulator (software version: 3.00), please refer to the Manual for the detailed operation steps.
- Some functional testers or patient simulators can measure the accuracy of the device copied calibration curve, but they can not be used to evaluate the device accuracy.
- When using the device, please keep it away from the equipment which can generate strong electric field or strong magnetic field. Using the device in an inappropriate environment may cause interference to the surrounding radio equipment or affect its working.
- The measured accuracy will be affected by the interference of electrosurgical equipment.
- When several products are used on the same patient simultaneously, danger may occur which is arisen from the overlap of leakage current.
- CO poisoning will appear excessive estimation, so it is not recommended to use the device.
- This device is not intended for treatment.
- The intended operator of the device may be a patient.
- Avoid maintaining the device during using.
Overview
The oxygen saturation is the percentage of HbO2 in the total Hb in the blood, so-called the O2 concentration in the blood, it is an important physiological parameter for the respiratory and circulatory system. A number of diseases related to respiratory system may cause the decrease of SpO2 in the blood, furthermore, some other causes such as the malfunction of human body’s self-adjustment, damages during surgery, and the injuries caused by some medical checkup would also lead to the difficulty of oxygen supply in human body, and the corresponding symptoms would appear as a consequence, such as vertigo, impotence, vomit etc. Serious symptoms might bring danger to human’s life. Therefore, prompt information of patients’ SpO2 is of great help for the doctor to discover the potential danger, and is of great importance in the clinical medical field. Insert the finger when measuring, the device will directly display the SpO2 value measured, it has a higher accuracy and repeatability.
Features
A. Easy to use.
B. Small in volume, light in weight, convenient to carry.
C. Low power consumption.
Applied range
The Pulse Oximeter can be used to measure human Hemoglobin Saturation and pulse rate through finger, and indicate the pulse intensity by the bar-display. The product is suitable for use in family, hospital(Ordinary sickroom ), Oxygen Bar, social medical organizations and also the measure of saturation oxygen and pulse rate.
Environment requirements
Storage Environment
- Temperature: -40 ℃ ~ + 60 ℃
- Relative humidity: ≤ 95%
- Atmospheric pressure: 500 hPa ~ 1060 hPa
Operating Environment
- Temperature: +10 ℃~ + 40 ℃
- Relative Humidity: ≤ 75%
- Atmospheric pressure: 700 hPa ~ 1060 hPa
Precautions
Attention
Point out conditions or practices that may cause damage to the device or other properties.
- Before using the device, make sure that it locates in normal working state and operating environment.
- In order to get a more accurate measurement, it should be used in a quiet and comfortable environment.
- When it is carried from cold environment to warm or humid environment, please do not use it immediately.
- If the device is splashed or coagulated by water, please stop operating.
- DO NOT operate the device with sharp things.
- High temperature, high pressure, gas sterilizing or immersion disinfection for the device is not permitted. Refer to User Manual in the relative chapter (6.1) for cleaning and disinfection..Please take out the internal battery before cleaning and disinfection.
- The device is suitable for children and adult.
- The device may not be suitable for all users, if you can’t get a satisfactory result, please stop using it.
- Data averaging and signal processing have a delay in the upgrade of SpO2 data values. When the data update period is less than 30 seconds, the time for obtaining dynamic average values will increase, which is arisen from signal degradation, low perfusion or other interference, it depends on the PR value.
- The device has 3-year service life, date of manufacture: see the label.
- The device hasn’t low-voltage prompt function, it only shows the low-voltage, please change the battery when the battery voltage is used up.
- The maximum temperature at the SpO2 probe-tissue interface should be less than 41℃ which is measured by the temperature tester.
- During measuring, when abnormal conditions appear on the screen, please pull out your finger and reinsert it to measure again.
- If some unknown error appears during measuring, remove the battery to terminate operating.
- Do not contort or drag the wire of the device.
- The plethysmographic waveform is not normalized, as a signal inadequacy indicator, when it is not smooth and stable, the accuracy of the measured value may degrade. When it tends to be smooth and stable, the measured value read is the optimal and the waveform at this time is also the most standard.
- If the device or component is intended for single-use, then the repeated use of these parts will pose risks on the parameters and technical parameters of the equipment known to the manufacturer.
- If necessary, our company can provide some information (such as circuit diagrams, component lists, illustrations, etc.), so that the qualified technical personnel of the user can repair the device components designated by our company.
- The measured results will be influenced by the external colouring agent (such as nail polish, colouring agent or color skin care products, etc.), so don’t use them on the test site.
- As to the fingers which are too cold or too thin or whose fingernail is too long, it may affect the measured results, so please insert the thicker finger such as thumb or middle finger deeply enough into the probe when measuring.
- The finger should be placed correctly (see Attached figure 5), as improper installation or improper contact position for sensor will influence the measurement.
- The light between the photoelectric receiving tube and the light-emitting tube of the device must pass through the subject’s arteriole. Make sure the optical path is free from any optical obstacles like rubberized fabric, to avoid inaccurate results.
- Excessive ambient light may affect the measured results, such as surgical light (especially xenon light sources), bilirubin lamp, fluorescent lamp, infrared heater and direct sunlight, etc. In order to prevent interference from ambient light, make sure to place the sensor properly and cover the sensor with opaque material.
- Frequent movement (active or passive) of the subject or severe activity can affect the measured accuracy.
- The Pulse Oximeter should not be placed on a limb with the blood pressure cuff, arterial ductus or intraluminal tube.
- The measured value may be inaccurate during defibrillation and in a short period after defibrillation, as it has not defibrillation function.
- The device has been calibrated before leaving factory.
- The device is calibrated to display functional oxygen saturation.
- The equipment connected with the Oximeter interface should comply with the requirements of IEC 60601-1.
Clinical restriction
- As the measure is taken on the basis of arteriole pulse, the substantial pulsating blood flow of subject is required. For a subject with weak pulse due to shock, low ambient/body temperature, major bleeding, or use of vascular contracting drug, the SpO2 waveform (PLETH) will decrease. In this case, the measurement will be more sensitive to interference.
- The measurement will be influenced by intravascular staining agents (such as indocyanine green or methylene blue), skin pigmentation.
- The measured value may be normal seemingly for the tester who has anemia or dysfunctional hemoglobin(such as carboxyhemoglobin (COHb), methemoglobin (MetHb) and sulfhaemoglobin (SuHb)), but the tester may appear hypoxia, it is recommended to perform further assessment according to the clinical situations and symptoms.
- Pulse oxygen only has a reference meaning for anemia and toxic hypoxia, as some severe anemia patients still show better pulse oxygen measured valued.
- Contraindication: no
Principle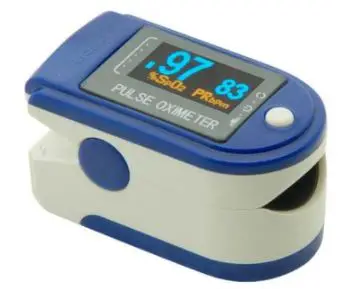
Principle of the Oximeter is as follows: An experience formula of data process is established taking use of Lambert Beer Law according to Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and Oxyhemoglobin (HbO2) in glow & near-infrared zones. Operation principle of the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning & Recording Technology, so that two beams of different wavelengths of lights can be focused onto human nail tip through perspective clamp finger-type sensor. Then measured signal can be obtained by a photosensitive element, information acquired through which will be shown on screen through treatment in electronic circuits and microprocessor.
Functions
- SpO2 value display
- PR value and bar graph display
- Low-battery indication: low-battery indication appears when the battery voltage is too low to work
- Automatic standby function
Installation
View of the front panel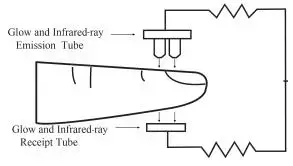
Battery
Step 1. Refer to Figure 3. and insert the two AAA size batteries properly in the right direction.
Step 2. Replace the cover.
Please take care when you insert the batteries for the improper insertion may damage the device.
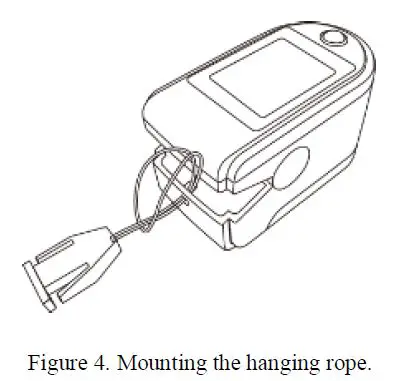
Mounting the hanging rope
Step 1. Put the end of the rope through the hole.
Step 2. Put another end of the rope through the first one and then tighten it.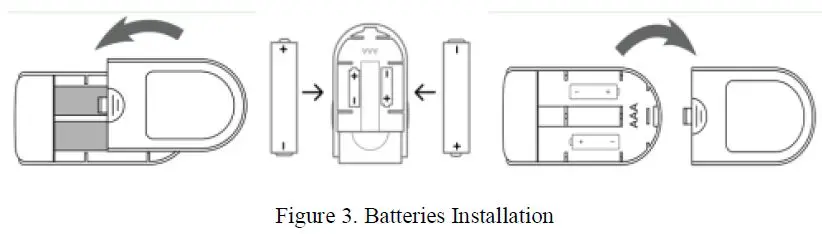
Structure, accessories and software description
- Structure: main unit.
- Accessories: one User Manual, one hanging rope. Please check the device and accessories according to the list to avoid that the device can not work normally.
- Software description
- Software name: CMS50DL embedded software
- Software specification: no
- Release version: V2.0
- Naming rule for version: V <Major enhancive software upgrade>.<Minor enhancive software upgrade>.<Improvement software upgrade> Involved algorithm: name: plethysmography; type: mature arithmetic
- Purpose: be used to measure SpO2, pulse rate, etc.
- Clinical function: calculate SpO2 and pulse rate values by collecting and processing the testee’ s pulse signal.
Operating Guide
- Insert the two batteries properly to the direction, and then replace the ncover.
- Open the clip as shown in Figure 5.
- Let the patient’s finger put into the rubber cushions of the clip (make sure the finger is in the right position), and then clip the finger.
- Press the button once on front panel.
- Do not shake the finger and keep the patient at ease during the process.Meanwhile, human body is not recommended in movement status.
- Get the information directly from screen display.
- In boot-strap state,press button ,and the device is reset.
Fingernails and the luminescent tube should be on the same side.
Maintain, Transport and Storage
Cleaning and disinfection
The device must be turned off before cleaning, and it should not be immersed into liquid. Please take out the internal battery before cleaning, do not immerse it into liquid. Use 75% alcohol to wipe the device enclosure, and use liquid soap or isopropanol to wipe the watchband for disinfection, nature dry or clean it with clean and soft cloth. Do not spray any liquid on the device directly, and avoid liquid penetrating into the device.
Maintenance
- Check the main unit and all accessories periodically to make sure that there is no visible damage that may affect patient’s safety and monitoring performance. It is recommended that the device should be inspected weekly at least. When there is obvious damage, stop using it.
- Please clean and disinfect the device before/after using it according to the User Manual (6.1).
- Please replace the batteries in time when low-battery appears.
- Please take out the batteries if the device is not used for a long time.
- The device need not to be calibrated during maintenance.
Transport and Storage
- The packed device can be transported by ordinary conveyance or according to transport contract. During transportation, avoid strong shock, vibration and splashing with rain or snow, and it can not be transported mixed with toxic, harmful, corrosive material.
- The packed device should be stored in room with no corrosive gases and good ventilation. Temperature: -40°C~+60°C; Relative humidity: ≤95%.
Troubleshooting
| Trouble | Possible Reason | Solution |
| The SpO2 and Pulse Rate can not be displayed normally | 1) The finger is not properly inserted.
2) The finger is shaking or the patient is moving. 3) The device is not used in environment required by the manual. 4) The device works abnormally. |
1) Please insert the finger properly and measure again.
2) Let the patient keep calm. 3) Please use the device in normal environment. 4) Please contact the after-sales. |
| The SpO2 and Pulse Rate are not displayed
stably |
1) The finger is not placed inside deep enough.
2) The finger is shaking or the patient is moving. |
1) Place the finger properly and try again.
2) Let the patient keep calm |
| The device can not be turned on | 1) The battery is drained away or almost drained away.
2) The battery is installed incorrectly. 3) The device’s malfunction. |
1) Please change batteries.
2) Please Install the battery again. 3) Please contact the local service center. |
| The display is off suddenly | 1) The device enters into the energy saving mode.
2) Low battery. 3) The device works abnormally. |
1) Normal.
2) Please charge the battery. 3) Please contact the after-sales. |
Key of Symbols
| Symbol | Description |
| Type BF | |
| Refer to instruction manual/booklet | |
| %SpO2 | The pulse oxygen saturation(%) |
| PRbpm | Pulse rate (bpm) |
| The battery voltage indication is deficient
(change the battery in time avoiding the inexact measure) |
|
| 1. No finger inserted
2. An indicator of signal inadequacy |
|
| Battery positive electrode | |
| Battery cathode | |
| Exit standby mode | |
| SN | Serial number |
| Alarm inhibit | |
| WEEE (2002/96/EC) | |
| IP22 | Ingress of liquids rank |
| Storage and Transport Temperature limitation | |
| Storage and Transport Humidity limitation |
| Storage and Transport Atmospheric pressure limitation | |
| This side up | |
| Fragile, handle with care | |
| Keep dry | |
| Recyclable | |
| European Representative | |
| This item is compliant with Directive 93/42/EEC of 14 june 1993 concerning medical devices; Including, at 21 march 2010, the amendments by Council Directive 2007/47/EEC. |
Note: Your device may not contain all the following symbols.
Function Specification
| SpO2 [see note 1] | |
| Display range | 0% ~ 99% |
| Measured range | 0% ~ 100% |
| Accuracy [see note 2] | 70%~100%: ±2%;
0%~69%: unspecified. |
| Resolution | 1% |
| PR | |
| Display range | 30 bpm ~ 250 bpm |
| Measured range | 30 bpm ~ 250 bpm |
| Accuracy | ±2 bpm or ±2%, whichever is greater. |
| Resolution | 1 bpm |
| Accuracy under low perfusion [see note 3] | Low perfusion 0.4%:
SpO2: ±4%; PR: ±2 bpm or ±2%, whi chever is greater |
|
Light interference |
Under normal and ambient light conditions, the SpO2 deviation ≤ 1% |
| Optical sensor [see note 4] | |
| Red light | Wavelength: about 660 nm, optical output power: < 6.65 mW |
| Infrared light | Wavelength: about 905 nm, optical output power: < 6.75 mW |
| Safety class | Internally powered equipment, type BF applied part |
| International Protection |
IP22 |
| Working voltage | DC 2.6 V ~ 3.6 V |
| Working current | ≤ 25 mA |
| Power supply | 1.5V (AAA size) alkaline batteries × 2 or rechargeable battery |
| Battery life | Two batteries can work continually for 24 hours |
| Dimension and Weight | |
| Dimension | 57 mm(L) × 31 mm(W) × 32 mm(H) |
| Weight | About 50 g (including a lithium battery) |
Note 1: the claims of SpO2 accuracy shall be supported by clinical study measurements taken over the full range. By artificial inducing, get the stable oxygen level to the range of 70 % to 100 % SpO2, compare the SpO2 values collected by the secondary standard pulse oximeter equipment and the tested equipment at the same time, to form paired data, which are used for the accuracy analysis. There are 12 healthy volunteers (male: 6. female: 6; age: 18~45; skin color: black: 2, light: 8, white: 2) data in the clinical report.
Note 2: because pulse oximeter equipment measurements are statistically distributed, only about two-thirds of pulse oximeter equipment measurements can be expected to fall within ±Arms of the value measured by a CO-OXIMETER.
Note 3: percentage modulation of infrared signal as the indication of pulsating signal strength, patient simulator has been used to verify its accuracy under conditions of low perfusion. SpO2 and PR values are different due to low signal conditions, compare them with the known SpO2 and PR values of input signal.
Note 4: optical sensors as the light-emitting components, will affect other medical devices applied the wavelength range. The information may be useful for the clinicians who carry out the optical treatment.For example, photodynamic therapy operated by clinician.
Note 5: Patient simulator has been used to verify the pulse rate accuracy, it is stated as the root-mean-square difference between the PR measurement value and the value set by simulator.
EMC
Note:
- The device is subject to special EMC precautions and it must be installed and used in accordance with these guidelines.
- The electromagnetic field can affect the device performance, so other equipment used near the device must meet the corresponding EMC requirements. Mobile phones, X-rays or MRI devices are possible interference source, as they can emit high-intensity electromagnetic radiation.
- Refer to above chapters for the minimum value of user’s physiological signal. Inaccurate result will appear when the device operates with the values lower than the descriptions in above chapter
- The use of ACCESSORIES, transducers and cables other than those specified, with the exception of transducers and cables sold by the MANUFACTURER of the ME EQUIPMENT or ME SYSTEM as replacement parts for internal components, may result in increased EMISSIONS or decreased IMMUNITY of the ME EQUIPMENT or ME SYSTEM.
- The device should not be used adjacent to or stacked with other equipment and that if adjacent or stacked use is necessary, it should be observed to verify normal operation in the configuration in which it will be used.
- Devices or systems may still be interfered by other equipment, even if other equipment meets the requirements of the corresponding national standard.
- Basic performance: SpO2 measured range: 70% ~ 100%, absolute error: ±2%; PR measured range: 30 bpm ~ 250 bpm, accuracy: ±2 bpm or ±2%, whichever is greater.
Appendix 1
Guidance and manufacture’s declaration Guidance and manufacture’s declaration – electromagnetic emissionsfor all EQUIPMENT and SYSTEMS
| Guidance and manufacture’s declaration – electromagnetic emission | ||
| The Pulse Oximeter is intended for use in the electromagnetic environment specified below. The customer of the user of the Pulse Oximeter should assure that it is used in such and
environment. |
||
| Emission test | Compliance | Electromagnetic environment – guidance |
| RF emissions CISPR 11 |
Group 1 |
The Pulse Oximeter uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any
interference in nearby electronic equipment. |
|
RF emission CISPR 11 |
Class B |
The Pulse Oximeter is suitable for use in all establishments, including domestic establishments and those directly connected to the public
low-voltage power supply network that supplies buildings used for domestic purposes. |
Guidance and manufacture’s declaration – electromagnetic immunity – for all EQUIPMENT and SYSTEMS
| Guidance and manufacture’s declaration – electromagnetic immunity | |||
| The Pulse Oximeter is intended for use in the electromagnetic environment specified below. The customer or the user of Pulse Oximeter should assure that it is used in such an
environment. |
|||
| Immunity test | IEC 60601
test level |
Compliance level | Electromagnetic environment – guidance |
|
Electrostatic discharge (ESD) IEC 61000-4-2 |
8 kV contact 15 kV air |
8 kV contact 15 kV air |
Floors should be wood, concrete or ceramic tile. If floor are covered with synthetic material, the relative humidity should be at least 30%. the manufacturer may recommend the ESD precautionary procedures to
user. |
| Power frequency (50Hz) magnetic field
IEC 61000-4-8 |
30A/m |
30A/m |
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital
environment. |
Guidance and manufacture’s declaration – electromagnetic immunity – for EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING
| Guidance and manufacture’s declaration – electromagnetic immunity | |||
| The Pulse Oximeter is intended for use in the electromagnetic environment specified below. The customer or the user of Pulse Oximeter should assure that it is used in such an
environment. |
|||
| Immunity test | IEC 60601
test level |
Complianc e level |
Electromagnetic environment – guidance |
|
Conducted |
3V(0.15MH |
3V(0.15M |
Portable and mobile RF communications equipment should be used no closer to any part of the Pulse Oximeter, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance d = é3.5 ù P ê V ú ë 1 û d = é3.5ù P 80 MHz to 800 MHz ê E ú ë 1 û d = é 7 ù P 800 MHz to 2.7 GHz ê E ú ë 1 û Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be less than the compliance level in each frequency range.b Interference may occur in the vicinity of equipment marked with the following symbol:
|
| RF | z–80MHz), | Hz–80MHz | |
| IEC | 6V(in ISM | ),6V(in | |
| 61000-4-6 | bands | ISM bands | |
| between | between | ||
| 0.15MHz | 0.15MHz | ||
| and | and | ||
| 80MHz) | 80MHz) | ||
| Radiated | |||
| RF | 10 V/m | ||
| IEC | 10 V/m | ||
| 61000-4-3 | 80 MHz to | ||
| 2.7GH | |||
| NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. |
|||
| a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Pulse Oximeter is used exceeds the applicable RF compliance level above, the Pulse Oximeter should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Pulse Oximeter.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m. |
|||
Recommended separation distances between portable and mobile RF communications equipment and the EQUIPMENT or SYSTEM – for EQUIPMENT or SYSTEM that are not LIFE-SUPPORTING.
| Recommended separation distances between
portable and mobile RF communications equipment and the Pulse Oximeter. |
|||
| The Pulse Oximeter is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Pulse Oximeter can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Pulse Oximeter as recommended below, according to the maximum output power of the
communications equipment. |
|||
| Rated | Separation distance according to frequency of transmitter(m) | ||
| maximum | 150 kHz to 80 MHz | 80 MHz to 800 MHz | 800 MHz to 2.7 GHz |
| output | |||
| power of transmitter (W) | d = é3.5 ù P
ê V ú ë 1 û |
d = é3.5ù P
ê E ú ë 1 û |
d = é 7 ù P
ê E ú ë 1 û |
| 0.01 | 0.058 | 0.035 | 0.07 |
| 0.1 | 0.18 | 0.11 | 0.22 |
| 1 | 0.58 | 0.35 | 0.7 |
| 10 | 1.83 | 1.10 | 2.21 |
| 100 | 5.8 | 3.5 | 7 |
| For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. |
|||
FCC Caution
Labeling requirements.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
- This device may not cause harmful interference, and
- this device must accept any interference received, including interference that may cause undesired operation.
Information to the user.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interfere nce to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase th e separation between the equipment and receiver.
- Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
- Consult the dealer or an experienced radio/TV technician for help.
15.21 Information to user.
Any Changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority to operate the equipment.